Thioglycosidic sterol glycoside and thioglycosidic stanol glycoside, preparation method and application thereof
A technology of thioglycosidic bond stanol glycosides and thioglycosidic bond sterol glycosides is applied in metabolic diseases, steroids, organic chemistry, etc., and can solve the problems of lack of access means, short drug half-life, and inhomogeneous structure. To achieve the effect of simplifying the experimental scheme, improving stability, and increasing flexibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
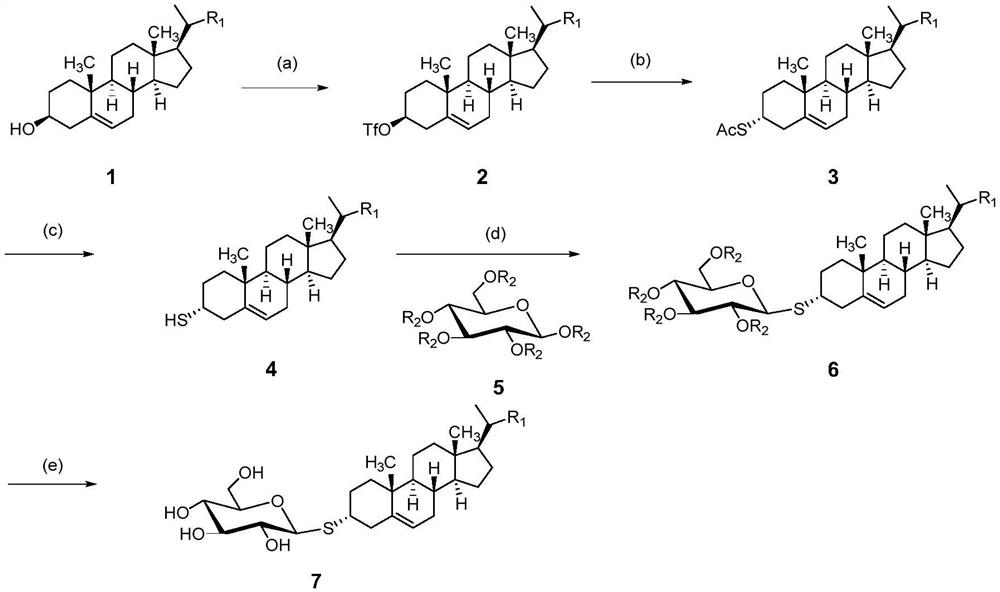
[0046] Rapeseed sterol (compound 1) was used as raw material and total acetyl protected glucose (compound 5) was used as sugar donor
[0047] Synthesis of compound 2
[0048] Dissolve crude natural sterol compound 1 (rapeseed sterol, 0.7G, 1.68mmol) in anhydrous DCM (10ml) and anhydrous pyridine (0.24ml) pre cooled to 0 ℃, and then slowly add anhydrous DCM (3.5ml) solution containing trifluoromethylsulfonic anhydride (1.422g, 2.8mmol) to the solution of the above compound 1 drop by drop. The dropping time needs to exceed 30min, and when adding DCM, it is necessary to ensure that the system is 0 ℃, After dropping, the reaction system needs to react continuously at 0 ℃ for 1 hour. After the reaction, wash with saturated sodium bicarbonate solution (50 ml) and magnesium sulfate (MgSO) 4 )After drying, filtration, evaporation and concentration, compound 2 (0.752g, 83%) substituted by trifluoromethylsulfonyl at position C-3 was obtained, 1H NMR (300MHz, DMSO) δ 1.24(m, 3H),1.90-1.65(m,...
Embodiment 2
[0058] Rapeseed oil stanol (compound 1) was used as raw material and total benzoyl protected glucose (compound 5) was used as sugar donor
[0059] Synthesis of compound 2
[0060] Dissolve crude natural sterol compound 1 (0.7G, 1.68mmol) in anhydrous DCM (10ml) and anhydrous pyridine (0.24ml) pre cooled to 0 ℃, and then slowly add anhydrous DCM (3.5ml) solution of trifluoromethyl sulfonic anhydride (1.422g, 2.8mmol) to the solution of compound 1 drop by drop. The dropping time needs to exceed 30min, and when DCM is added, it needs to ensure that the system is 0 ℃, and the reaction system needs to react continuously at 0 ℃ for 1 hour after dropping. After the reaction, wash with saturated sodium bicarbonate solution (50ml) and magnesium sulfate (MgSO 4 )After drying, filtration, evaporation and concentration, compound 2 (0.730g, 81%) substituted by trifluoromethylsulfonyl at position C-3 was obtained, 1H NMR (300MHz, DMSO) δ 1.24(m,3H),1.90-1.65(m,2H),1.90-1.65(m,2H),4.73(m,1H),1.5...
Embodiment 3
[0070] The preparation method of example 3 is the same as that of example 1, except that compound 1 is rapeseed sterol, and the amount of trifluoromethyl sulfonic anhydride in step (1) is twice the number of moles of compound 1; The reaction was carried out at - 10 ℃; In step (2), the amount of potassium thioacetate is 1 times the number of moles of compound 2; The reaction was carried out at - 20 ℃; The reaction solvent used in step (3) is ethyl acetate; The reaction reagent used is potassium hydroxide, and the amount of basic reagent is 5 times the number of moles of compound 3; The reaction solvent used in step (4) is toluene, the Lewis acid catalyst used is trifluoromethane sulfonic acid, and the amount of Lewis acid catalyst is 0.5 times the number of moles of compound 3; The inert shielding gas is helium; In step (5), compound 6 is hydrolyzed and deprotected with alkali. The alkali is potassium hydroxide (KOH), the reaction solvent is ether, and the amount of alkaline reagen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com