Application of nano metal oxide in catalyzing persulfate to degrade organic dyes
A nano metal, persulfate technology, applied in metal/metal oxide/metal hydroxide catalyst, chemical/physical process, physical/chemical process catalyst, etc., can solve the problem of low catalytic performance, difficult practical application, no priority Crystal plane orientation and other issues, to achieve the effect of less dosage, excellent degradation ability and outstanding performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
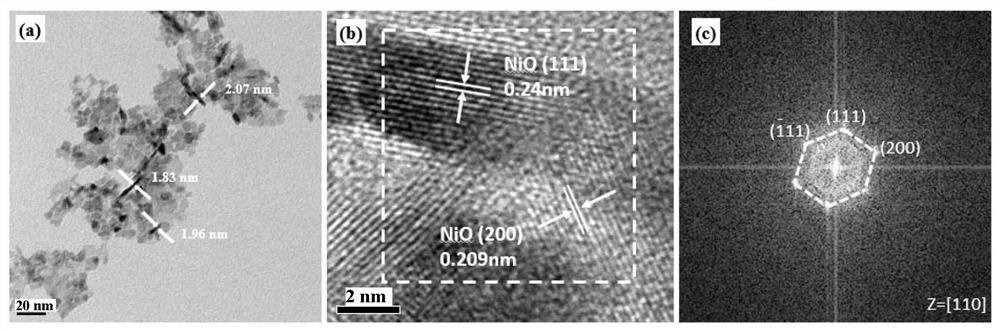
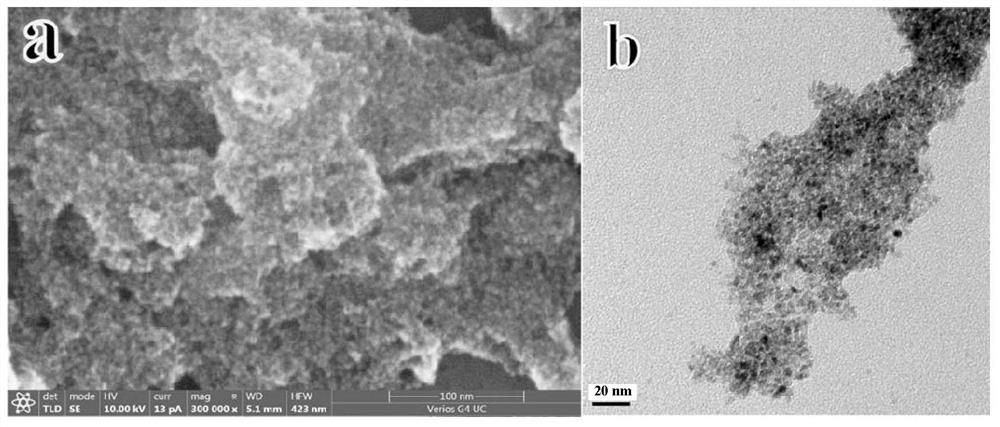
[0046] 2.5g cupric chloride dihydrate was ground in a mortar for five minutes, then 0.6g sodium hydroxide and 0.8g sodium carbonate were ground for five minutes, then mixed together and ground for thirty minutes to obtain a catalyst precursor, after centrifugal washing three times, Dry at 60°C for 24 hours, then keep at 120°C for 8 hours, put it into a muffle furnace and bake at 400°C for 2 hours, and finally obtain the copper oxide catalyst used in this experiment. The obtained sample is in the form of brown powder. figure 1 The SEM photo of the prepared copper oxide shows that the particle size of the copper oxide is 30-80nm. figure 2 is the XRD pattern of the prepared copper oxide.
[0047] Add 30 mg of the above-mentioned copper oxide catalyst into 100 ml of an aqueous solution containing 50 mg of potassium peroxodisulfate and 50 mg of rhodamine B, react at room temperature for a certain period of time under stirring, use a syringe to pipette 5 ml of the reaction solution...
Embodiment 2
[0049] 2.5g cupric chloride dihydrate was ground in a mortar for five minutes, then 0.6g sodium hydroxide and 0.8g sodium carbonate were ground for five minutes, then mixed together and ground for thirty minutes to obtain a catalyst precursor, after centrifugal washing three times, Dry at 60°C for 24 hours, then keep at 120°C for 8 hours, put it into a muffle furnace and bake at 400°C for 2 hours, and finally get the copper oxide catalyst used in this experiment. The obtained sample is in the form of brown powder. The crystal face (110) is the main one.
[0050] Add 30 mg of the above-mentioned copper oxide catalyst into 100 ml of an aqueous solution containing 50 mg of potassium persulfate (Oxone) and 50 mg of rhodamine B, and react under stirring. After reacting for a certain period of time, use a syringe to pipette 5ml of the reaction solution, filter the solid catalyst quickly and measure its absorbance at a wavelength of 540nm, and calculate the degradation rate of rhodam...
Embodiment 3
[0052] 1.5 g of ferrous sulfate heptahydrate was ground in a mortar for five minutes, then 3 g of ferric chloride hexahydrate was ground for five minutes, then 0.8 g of sodium hydroxide was ground for five minutes, then mixed together and ground for thirty minutes to obtain a catalyst precursor The body was washed by centrifugation for three times, dried at 60°C for 24 hours, kept at 120°C for 8 hours, put into a muffle furnace and roasted at 400°C for 2 hours, and finally obtained the ferric oxide catalyst used in this experiment. The sample is in the form of brown powder, mainly exposed high-energy crystal faces (311), and the particle size is 20-60nm. image 3 is the XRD pattern of the prepared ferric oxide.
[0053] Add 30 mg of the above ferric oxide catalyst into 100 ml of an aqueous solution containing 50 mg of potassium peroxodisulfate and 50 mg of rhodamine B, and react under stirring. After reacting for a certain period of time, use a syringe to pipette 5ml of the r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com