Method for preparing ruthenium nitrosyl nitrate solution
A technology of ruthenium nitrosyl nitrate and nitric acid aqueous solution, applied in chemical instruments and methods, ruthenium/rhodium/palladium/osmium/iridium/platinum compounds, inorganic chemistry, etc., can solve the problem of large reaction mass transfer resistance, nitrosylation Low degree, increased preparation complexity and other issues, to achieve a high degree of nitrosylation, improve the degree of nitrosylation of ruthenium, and low chlorine content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
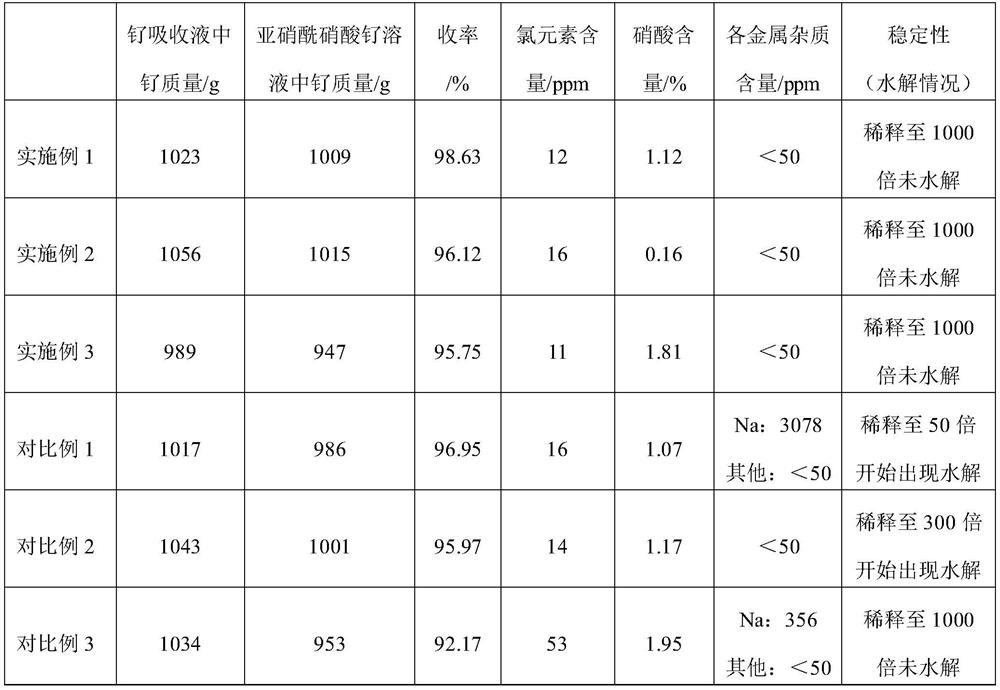
[0042] (1) adopt chlorine oxidative distillation method to make ruthenium tetroxide gas, pass into nitric acid massfraction then and carry out absorption reaction in the nitric acid aqueous solution (containing nitric acid 5000g) of 65%, reaction temperature is 80 ℃, when ruthenium ion absorption reaches in solution At about 1000 g, stop feeding ruthenium tetroxide gas to prepare ruthenium absorbing liquid. Use the inductively coupled plasma spectrometer (ICP) to measure the mass of ruthenium ion in the ruthenium absorption solution, denoted as M 1 , measured M 1 = 1023 g.
[0043] (2) Lower the temperature of the ruthenium absorption solution prepared in step (1) to room temperature, then use a water bath to further lower the temperature to 10°C, add 300g (10mol) of formaldehyde at a speed of 20mL / min under stirring conditions, and react for 10h after the addition, and prepare Nitrosyl-containing ruthenium absorption solution.
[0044] (3) In the ruthenium absorption solut...
Embodiment 2
[0047] (1) adopt chlorine oxidative distillation method to make ruthenium tetroxide gas, pass into nitric acid massfraction then and carry out absorption reaction in the nitric acid aqueous solution (containing nitric acid 3000g) of 45%, reaction temperature is 95 ℃, when ruthenium ion absorption reaches in solution At about 1000 g, stop feeding ruthenium tetroxide gas to prepare ruthenium absorbing liquid. Use ICP to measure the mass of ruthenium ions in the ruthenium absorption solution, denoted as M 1 , measured M 1 = 1056 g.
[0048] (2) Lower the temperature of the ruthenium absorbing solution prepared in step (1) to room temperature, then use a water bath to further cool down to 15°C, add an aqueous solution containing 540g (6mol) oxalic acid at a speed of 5mL / min under stirring, and complete the reaction 4h, the ruthenium absorption solution containing nitrosyl was prepared.
[0049] (3) In the nitrosyl-containing ruthenium absorption solution prepared in step (2), s...
Embodiment 3
[0052] (1) adopt chlorine oxidative distillation method to make ruthenium tetroxide gas, pass into nitric acid massfraction then and carry out absorption reaction in the nitric acid aqueous solution (containing nitric acid 8000g) of 50%, reaction temperature is 60 ℃, when ruthenium ion absorption reaches in solution At about 1000 g, stop feeding ruthenium tetroxide gas to prepare ruthenium absorbing liquid. Use ICP to measure the mass of ruthenium ions in the ruthenium absorption solution, denoted as M 1 , measured M 1 =989g.
[0053] (2) Lower the temperature of the ruthenium absorption solution prepared in step (1) to room temperature, then use a water bath to further lower the temperature to 5°C, add 1380g (30mol) of ethanol at a speed of 50mL / min under stirring, and react for 6h after adding, A ruthenium absorption solution containing nitrosyl was prepared.
[0054] (3) In the nitrosyl-containing ruthenium absorption solution prepared in step (2), slowly add an aqueous ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com