Preparation method of 2,5-dimethoxyphenylacetic acid
A technology of dimethoxybenzene and dimethoxyphenyl, which is applied in the field of drug synthesis, can solve the problems of good safety, difficulty in meeting market demand, and high cost, and achieve an effect that is conducive to environmental protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
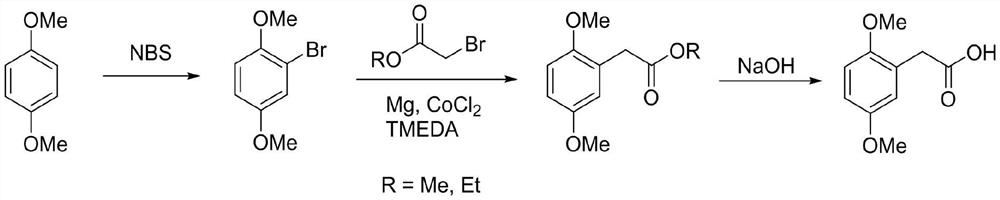
[0025] A. Add 9.96g (0.07208mol) of 1,4-dimethoxybenzene, 13.47g (0.07568mol) of N-bromosuccinimide, AuCl 3 (0.003mmol), dichloromethane 80mL, the mixture was stirred and reacted at 25°C for 2 hours, 40mL of water was added to the reaction system and stirred for 30min, the liquid was separated, the aqueous phase was extracted once with 40mL of dichloromethane, the organic phase was combined, anhydrous sodium sulfate Dry, concentrate the organic phase under reduced pressure, concentrate the residue and distill under reduced pressure to obtain 15.3 g of colorless liquid 2-bromo-1,4-dimethoxybenzene, yield 98%, HPLC 98.2%;
[0026] B. Add 10.85g (0.05mol) of 2-bromo-1,4-dimethoxybenzene, 7.64g (0.05mol) of methyl bromoacetate, and CoCl 2 65mg (0.5mmol), 1.44g (0.06mol) of magnesium strips, 116mg (1mmol) of N,N,N',N'-tetramethylethylenediamine, 250mL of anhydrous tetrahydrofuran, stirred at 25°C under the protection of argon React for 2 hours, add saturated ammonium chloride sol...
Embodiment 2
[0031] A. Add 9.96g (0.07208mol) of 1,4-dimethoxybenzene, 14.11g (0.07928mol) of N-bromosuccinimide, AuCl 3 (0.003mmol), chloroform 80mL, the mixture was stirred and reacted at 30°C for 1.5 hours, 40mL of water was added to the reaction system and stirred for 30min, the liquid was separated, the aqueous phase was extracted once with 40mL of dichloromethane, the organic phase was combined, anhydrous sodium sulfate Dry, concentrate the organic phase under reduced pressure, concentrate the residue and distill under reduced pressure to obtain 15.17 g of colorless liquid 2-bromo-1,4-dimethoxybenzene, yield 97%, HPLC 98.2%;
[0032] B. Add 10.85g (0.05mol) of 2-bromo-1,4-dimethoxybenzene, 8.4g (0.055mol) of methyl bromoacetate, and CoCl 2 65mg (0.5mmol), 1.44g (0.06mol) of magnesium strips, 116mg (1mmol) of N,N,N',N'-tetramethylethylenediamine, 250mL of anhydrous tetrahydrofuran, stirred at 30°C under the protection of argon React for 3 hours, add saturated ammonium chloride solut...
Embodiment 3
[0037] A. Add 9.96g (0.07208mol) of 1,4-dimethoxybenzene, 15.39g (0.08649mol) of N-bromosuccinimide, AuCl 3 (0.003mmol), dichloromethane 80mL, the mixture was stirred and reacted at 20°C for 3 hours, 40mL of water was added to the reaction system and stirred for 30min, the liquid was separated, the aqueous phase was extracted once with 40mL of dichloromethane, the organic phase was combined, anhydrous sodium sulfate Dry, concentrate the organic phase under reduced pressure, concentrate the residue and distill under reduced pressure to obtain 15.24 g of colorless liquid 2-bromo-1,4-dimethoxybenzene, yield 97.5%, HPLC 98.2%;
[0038] B. Add 10.85g (0.05mol) of 2-bromo-1,4-dimethoxybenzene, 9.17g (0.06mol) of methyl bromoacetate, and CoCl 2 65mg (0.5mmol), 1.44g (0.06mol) of magnesium strips, 116mg (1mmol) of N,N,N',N'-tetramethylethylenediamine, 250mL of anhydrous tetrahydrofuran, stirred at 35°C under the protection of argon React for 3 hours, add saturated ammonium chloride ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com