Preparation method of chiral bisoprolol fumarate
A bisoprolol fumarate and chirality technology is applied in the field of preparation of chiral bisoprolol fumarate intermediates and chiral bisoprolol fumarate, which can solve the problems of low purity of a single configuration and achieve the Conducive to industrialized production, simple process and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
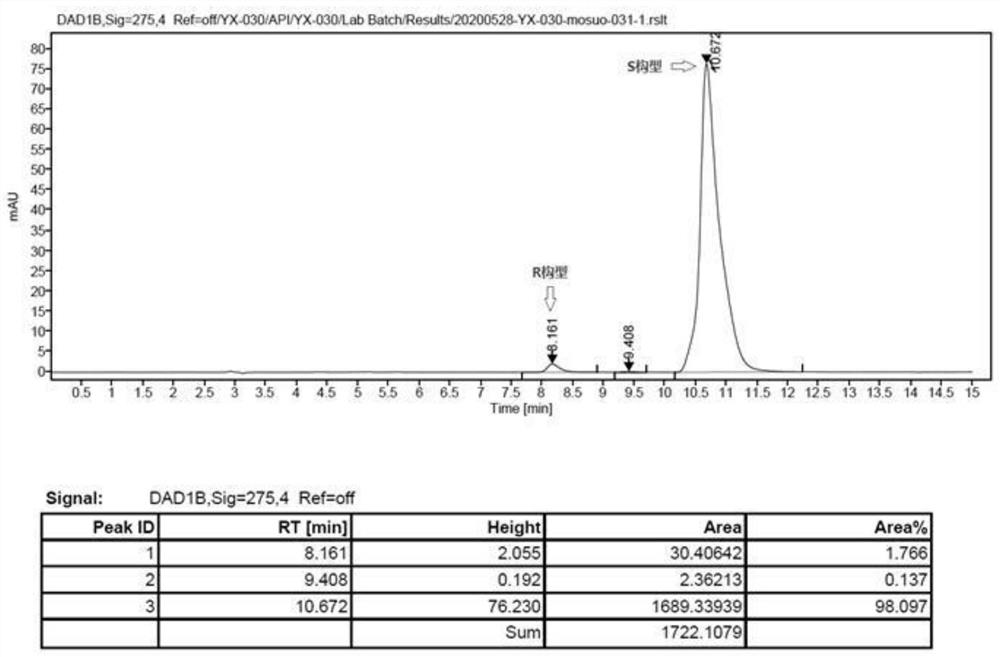
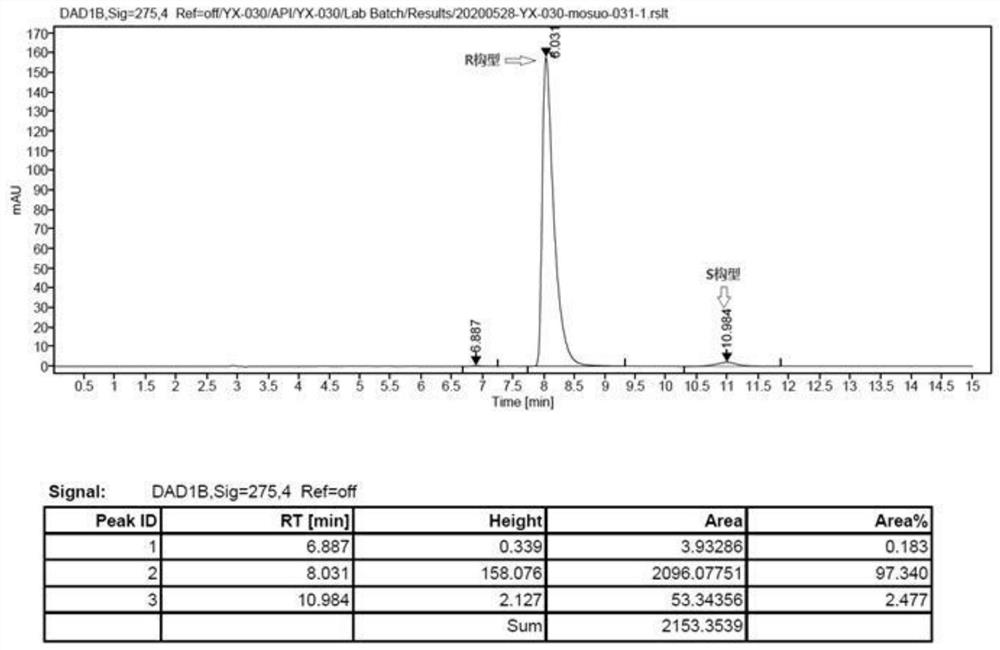
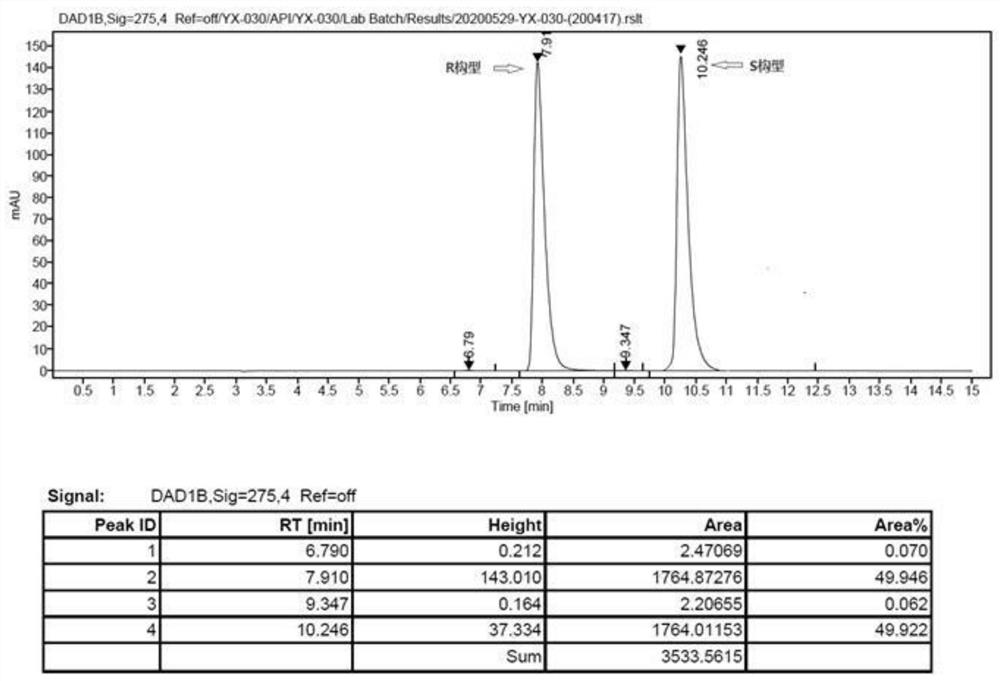
Image
Examples
Embodiment 1
[0035] Preparation of 4-((2-isopropoxyethoxy)methyl)phenyl mesylate
[0036]
[0037] Add 21.1g (100mmol) of p-isopropoxyethoxymethylphenol I and 250ml of acetonitrile into a 500ml three-necked flask, stir until the system is completely dissolved and drop below 10°C, add 11.5g (100mmol) of methylsulfonyl chloride in batches ), the temperature was raised to 60° C., and the reaction was continued for 4 hours, and the reaction was detected by TLC. The reaction solution was washed with 10% sodium bicarbonate solution and water, and dried over anhydrous sodium sulfate. The solvent was recovered under reduced pressure to obtain 26.4 g of 4-((2-isopropoxyethoxy)methyl)phenyl methanesulfonate III as a white solid, with a yield of 91.6%.
[0038] ESI-MS (m / z): [M+H] 290.1.
[0039] 1 HNMR (500MHz, DMSO-d6, δppm): 1.13-1.14 (d, 6H), 3.52-3.56 (m, 4H), 3.58 (s, 3H), 3.67 (m, 1H), 4.82 (s, 2H), 7.06 (d, 2H), 7.32 (d, 2H).
Embodiment 2
[0041] Preparation of (S)-2-((4-((2-isopropoxyethoxy)methyl)phenoxy)methyl)oxirane
[0042]
[0043] Add 14.4g (50mmol) of 4-((2-isopropoxyethoxy)methyl)phenyl mesylate, 5g (50mmol) of triethylamine and 200mL of toluene into a 500ml reaction flask, and stir until the system is completely dissolve. 3.7 g (50 mmol) of R-glycidyl alcohol was slowly added, and the reaction was continued at 70° C. for 2 hours, and the reaction was detected by TLC. The reaction solution was poured into 50 mL of ice water, extracted with toluene, and the organic phase was dried and distilled. The residue was 12.8 g of oil, yield 96.2%.
[0044] ESI-MS (m / z): [M+H] 267.2
[0045] 1 H-NMR (CDCl3, δppm), δ: 1.17-1.78 (d, 6H, 2CH3), 2.74-2.75 (dd, 1H), 2.90-2.91 (dd, 1H), 3.35-3.36 (m, 1H), 3.60 (s, 4H), 3.70 (s, 1H), 3.97-3.98 (dd, 1H), 4.20-4.21 (dd, 1H), 4.51 (s, 2H), 6.88 and 7.28 (dd, 4H).
Embodiment 3
[0047] Preparation of (S)-bisoprolol:
[0048]
[0049] In a 100mL three-necked flask, 5.30g (0.02mol) of compound (S)-2-((4-((2-isopropoxyethoxy)methyl)phenoxy)methyl)oxirane Dissolve in 20mL of methanol, add 20mL of isopropylamine, stir and react at 40-45°C for 3 hours, distill to dryness under reduced pressure, wash with water, extract with toluene, dry the organic phase, and distill to obtain 6.15g of light yellow oil (S)-bisoprol Er, the yield is 97.2%.
[0050] ESI-MS(m / z): [M+H]326.3
[0051] 1 H-NMR (CDCl3, δppm), δ: 1.15-1.16 (d, 6H), 1.17-1.18 (d, 6H), 2.88-2.90 (m, 3H), 3.61-3.62 (m, 4H), 3.95-3.96 (m, 2H), 3.96 (s, 2H), 4.02-4.03 (m, 1H, NH), 6.87-6.88 (d, 2H), 7.26-.27 (d, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com