A kind of preparation method of hydroxyl polyurethane
A technology of hydroxyl polyurethane and prepolymers, which is applied in the field of polymer material synthesis, can solve the problems of large-scale industrial production that is not suitable for PHU, and achieve good practical significance and application prospects, high product yield, and wide application range.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
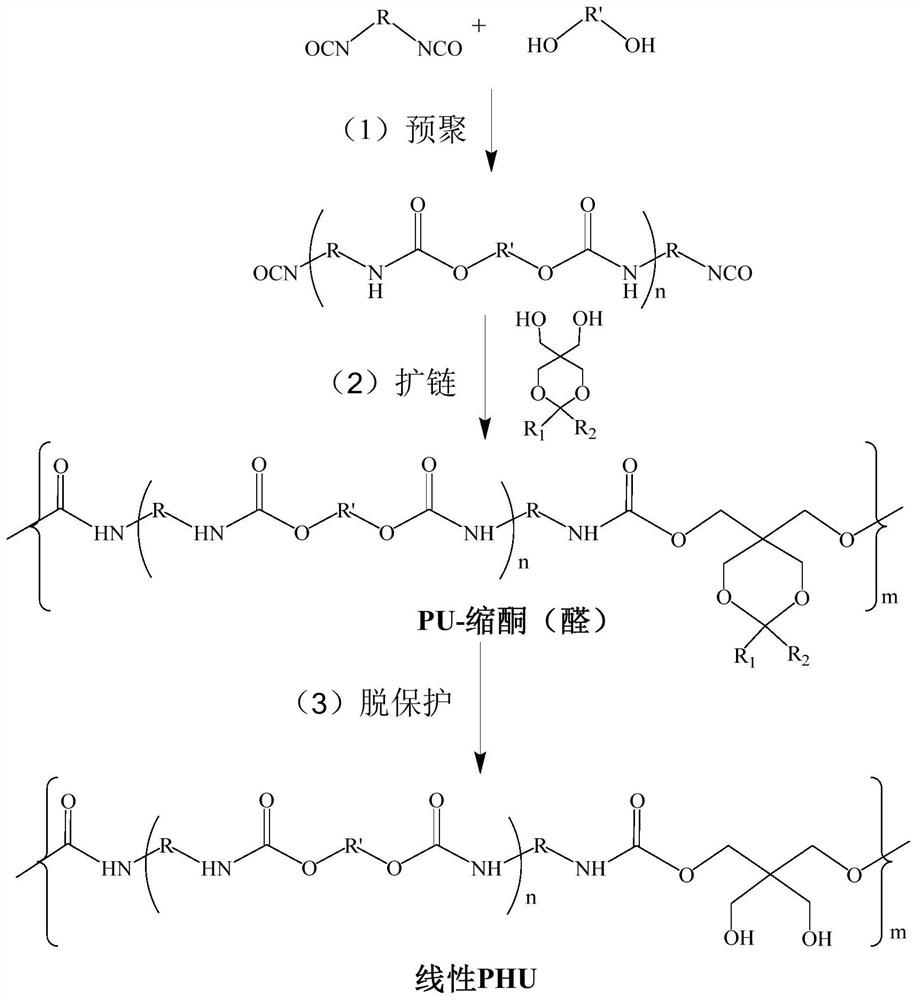
[0059] A kind of preparation of linear PHU, preparation route such as figure 1 As shown, the specific steps are as follows:
[0060] (1) Preparation of pentaerythritol monoketal:
[0061] Dissolve 30.00g of pentaerythritol and 0.42g of p-toluenesulfonic acid monohydrate in DMF at 80°C, let it stand and cool to 40°C, add 22.95g of 2,2-dimethoxypropane dropwise, and the mixture is cooled at 25°C Stir for 24 hours, add 0.13g trimethylamine and continue to stir for 2 hours, remove DMF by rotary evaporation, dissolve the crude product in dichloromethane, wash three times with deionized water, collect the organic layer, add anhydrous magnesium sulfate to dry, and remove dichloromethane by rotary evaporation. Chloromethane, dried in a vacuum oven at 40°C for 6 hours to obtain powdered pentaerythritol monoketal.
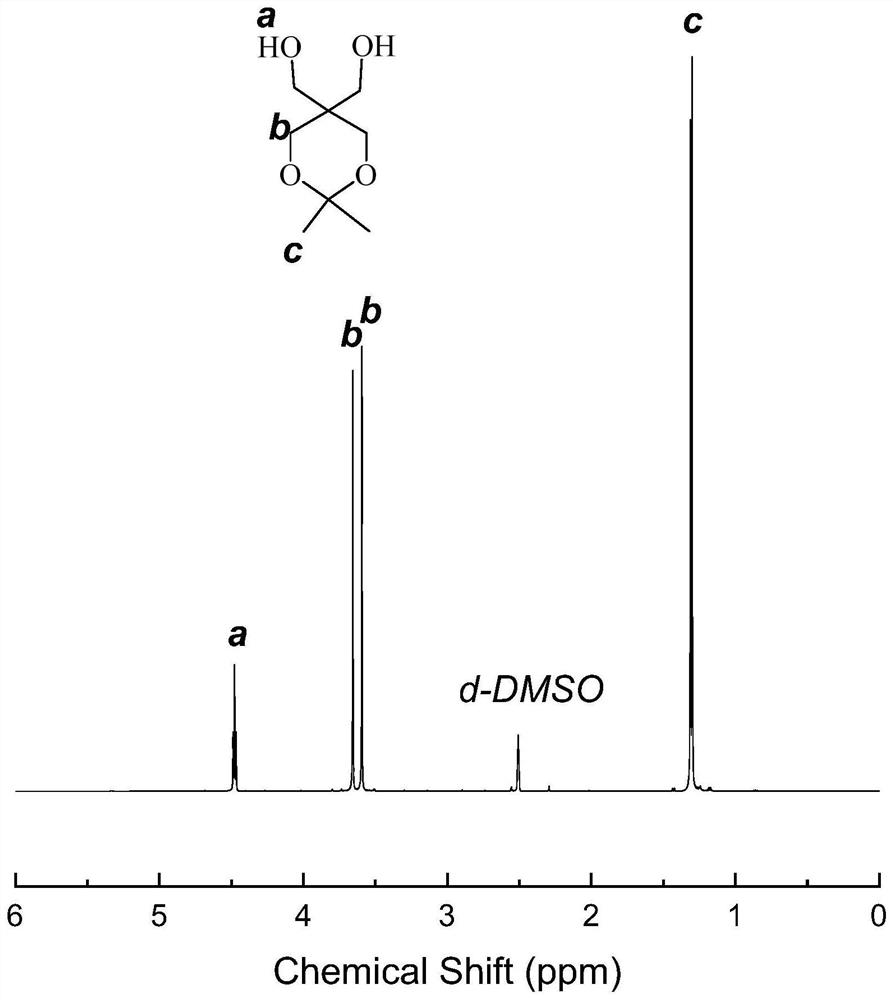
[0062] image 3 It is the proton nuclear magnetic resonance spectrogram of the pentaerythritol monoketal prepared. Specifically, 4.48ppm is the proton peak on the hydrox...
Embodiment 2
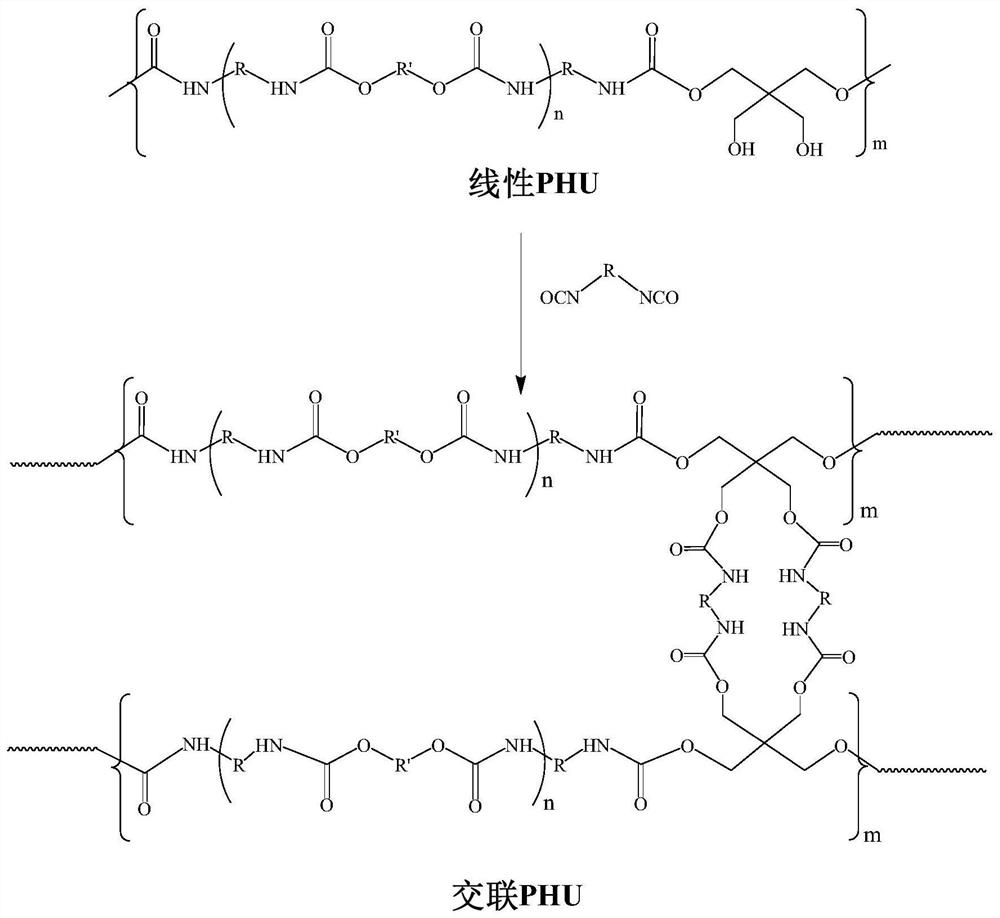
[0074] Preparation of cross-linked M-PHU: The synthetic route is shown in figure 2 As shown, the specific steps are as follows:
[0075] The linear M-PHU that 10.00g embodiment 1 is made and 0.75g MDI are dissolved in DMF, join in the reactor that mechanical stirring and nitrogen device are housed, then add dibutyltin dilaurate (accounting for all The mass ratio of reactants is 0.05%), and after passing nitrogen into the system for 0.5 h, the reaction is carried out at 60° C. until a gel is formed. After the gel was taken out, it was placed in an oven at 60°C for 12 hours to remove most of the solvent, and the remaining small amount of solvent was completely removed in a vacuum oven at 60°C for 48 hours to obtain a cross-linked M-PHU material.
[0076] Figure 7 For the photo of the reprocessing process of the cross-linked M-PHU material, the block sample of the cross-linked M-PHU was selected and cut into pieces. It was found that under certain temperature and pressure con...
Embodiment 3
[0079] (1) Preparation of pentaerythritol monoketal:
[0080] Dissolve 30.00g of pentaerythritol and 0.42g of p-toluenesulfonic acid monohydrate in DMF at 80°C, let it stand and cool to 40°C, add 22.95g of 2,2-dimethoxypropane dropwise, and the mixture is cooled at 25°C Stir for 24 hours, add 0.13g trimethylamine and continue to stir for 2 hours, remove DMF by rotary evaporation, dissolve the crude product in dichloromethane, wash three times with deionized water, collect the organic layer, add anhydrous magnesium sulfate to dry, and remove dichloromethane by rotary evaporation. Chloromethane, dried in a vacuum oven at 40°C for 6 hours to obtain powdered pentaerythritol monoketal.
[0081] (2) Preparation of isocyanate-terminated prepolymer:
[0082]Add 10.00g of diphenylmethane diisocyanate (MDI) and 26.67g of polytetrahydrofuran diol (PTMG, Mn=1,000Da) into a reaction kettle equipped with mechanical stirring and a nitrogen device, and then add dilauric acid di Butyltin (ac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| breaking strength | aaaaa | aaaaa |
| breaking strength | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com