Nano coronavirus recombinant vaccine taking graphene oxide as carrier
A coronavirus and graphene technology, applied in the fields of nanomaterials and biomedicine, can solve the problems of vaccine development failure, inability to produce immune memory, vaccine approval and marketing, etc., and achieve strong immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Preparation process of graphene oxide (GO)-carnosine-CpG-RBD recombinant protein vaccine preparation
[0045] The TLR9 receptor nucleic acid sequence CpG ODN M362, which has cross-reactivity to both humans and mice, is selected. The specific sequence is as follows: 5'-TCGTCGTCGTTC:GAACGACGTTGAT-3' (25 mer, SEQ ID NO 1), using the improved method of EDC-NHS reaction For the coupling process of GO and carnosine, 26 mg of GO freeze-dried powder was added to 5.20 mL of phosphate buffered saline (PBS, pH = 7.4), and sonicated (200 W, 40 kHz) at 25°C for 3 h . Add 6.82 mg EDC (N1-((ethylimino)methylene)-N3,N3-dimethylpropane-1,3-diamine at 25°C, Chinese: 1-(3-dimethylaminopropyl)-3-ethylcarbon diimide) and 7.73 mg NHS (N-N-hydroxysuccinimide) to activate the GO solution. Excess EDC / sulfo-NHS was removed from the reaction solution by ultrafiltration, and then the pH of the solution was adjusted to 7.4. Then, 40 mg of carnosine, 1.2ug of CpG, and different concentrations of ...
Embodiment 2
[0047] Graphene oxide (GO)-carnosine-CpG-RBD recombinant protein vaccine immunization experiments in mice
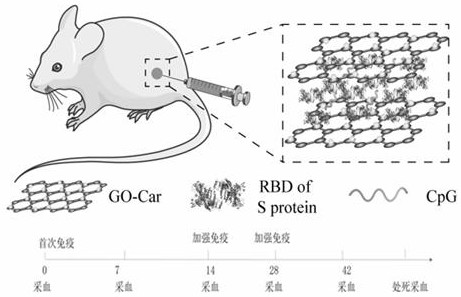
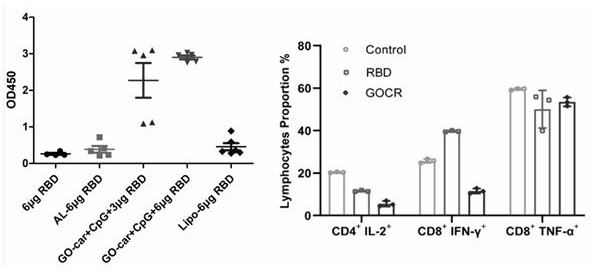
[0048] according to figure 1 The marked schedules were immunized with 6-week-old female BALB / cmice mice by subcutaneous injection on days 0, 14, and 28, until day 28 and day 42. Blood was collected by subcutaneous injection, serum was separated, and serum was detected. Specific antibodies against RBD. Mice were sacrificed on day 42, splenocytes were isolated, and the specific T cell immune response and cytokine secretion were detected.
[0049] Grouping and dose determination of immunized mice:
[0050] 1. (Graphene oxide+carnosine)+ 1.2ug cpG+3ug RBD
[0051] 2. (Graphene oxide+carnosine)+ 1.2ug cpG+6ug RBD
[0052] 3. Aluminum hydroxide + 6ug RBD (1:1)
[0053] 4. 6ug RBD
[0054] 5. Liposome (lipo) + 6ug RBD group
[0055] Mouse strain: BALB / c mice (n=6).
[0056] The schedule for immunizing mice with GO-Car-carnosine-CpG-RBD vaccine is: blood collection and f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com