N-type polymer containing non-condensed ring receptor unit as well as preparation method and application of n-type polymer
A technology of acceptor units and polymers, which is applied in the field of n-type polymers and their preparation, can solve the problems of high synthesis cost, low absorption coefficient, and low synthesis cost, and achieve enhanced charge transfer effect, high absorption coefficient, and manufacturing low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
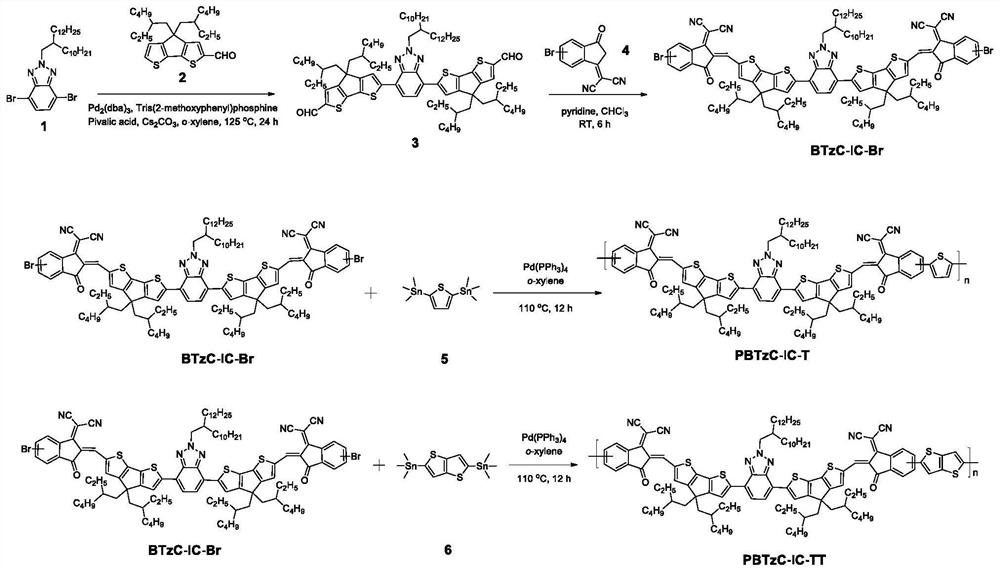
[0054] Preparation of polymerized monomer BTzC-IC-Br
[0055] The chemical synthesis route of BTzC-IC-Br is shown below, and the specific reaction steps and reaction conditions are as follows:
[0056]
[0057] (1) Raw materials 1 and 2 and terminal compound 4 were prepared according to the methods reported in the literature (refer to the literature for the synthesis of raw material 1: J.Mater.Chem.C, 2015, 3, 2792-2797; ACS Appl.Polym.Mater ., 2019, 1, 2302-2312. References for the synthesis of starting material 2: Small Methods, 2019, 1900531; ACS Appl. Mater. Interfaces, 2020, 12, 16531-16540. References for the synthesis of compound 4: Eur.J.Org .Chem., 2016, 2404-2412; J.Am.Chem.Soc., 2017, 139, 1336-1343), other reagents required for each step of the reaction were purchased from commercial channels.
[0058] (2) Synthesis of compound 3
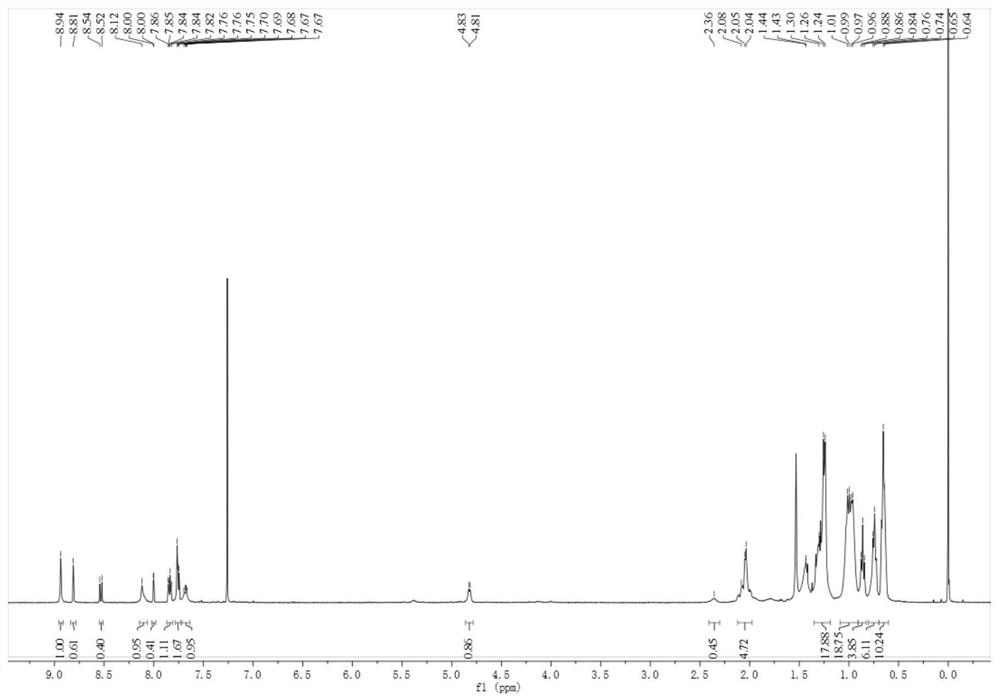
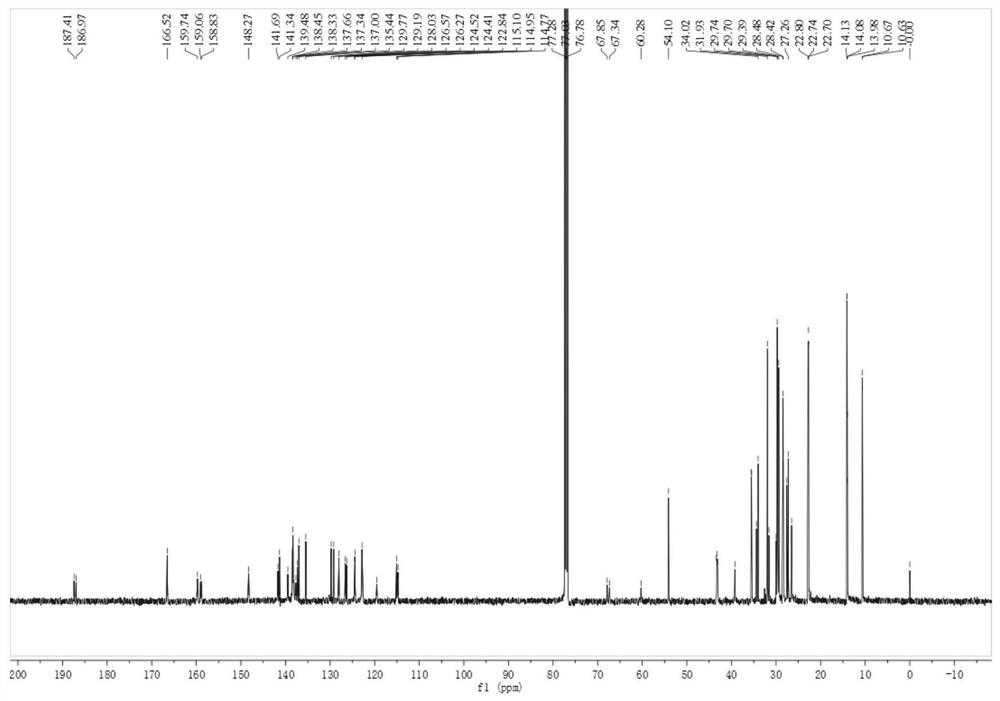
[0059] Add raw material 1 (325 mg), raw material 2 (500 mg), pivalic acid (54 mg), cesium carbonate (138 mg), three (o-methoxyphen...
Embodiment 2
[0063] Preparation of Polymer PBTzC-IC-T
[0064] The chemical synthesis route of PBTzC-IC-T is shown below, and the specific reaction steps and reaction conditions are as follows:
[0065]
[0066] (1) Polymerization monomer 5, catalyst tetrakis(triphenylphosphine) palladium and o-xylene were purchased from commercial channels.
[0067] (2) Synthesis of polymer PBTzC-IC-T
[0068] Monomer BTzC-IC-Br (0.10mmol, 182.33mg), monomer 5 (0.10mmol, 40.98mg) were dissolved in o-xylene (5mL), and tetrakis(triphenylphosphine) palladium (0.002mmol, 2.31 mg), after being degassed by nitrogen gas, stirred at 110°C for 12 hours, cooled to room temperature, added 2-(tributyltin)thiophene for 2 hours, and then added 2-bromothiophene for 2 hours. After cooling to room temperature, the polymer was precipitated in methanol, then placed in a Soxhlet extractor, extracted with methanol, acetone, n-hexane, and dichloromethane, and finally concentrated the obtained dichloromethane components, ...
Embodiment 3
[0070] Preparation of Polymer PBTzC-IC-TT
[0071] The chemical synthesis route of PBTzC-IC-TT is shown below, and the specific reaction steps and reaction conditions are as follows:
[0072]
[0073] (1) Polymerized monomer 6, catalyst tetrakis(triphenylphosphine)palladium and ortho-xylene were purchased from commercial channels.
[0074] (2) Synthesis of polymer PBTzC-IC-TT
[0075] Monomer BTzC-IC-Br (0.08mmol, 145.86mg), monomer 6 (0.08mmol, 37.27mg) were dissolved in o-xylene (4mL), and tetrakis(triphenylphosphine) palladium (0.0016mmol, 1.85 mg), after being degassed by nitrogen gas, stirred at 110°C for 12 hours, cooled to room temperature, added 2-(tributyltin)thiophene for 2 hours, and then added 2-bromothiophene for 2 hours. After cooling to room temperature, the polymer was precipitated in methanol, then placed in a Soxhlet extractor, extracted with methanol, acetone, n-hexane, dichloromethane, and chloroform, and the residue was dissolved by heating with chlor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com