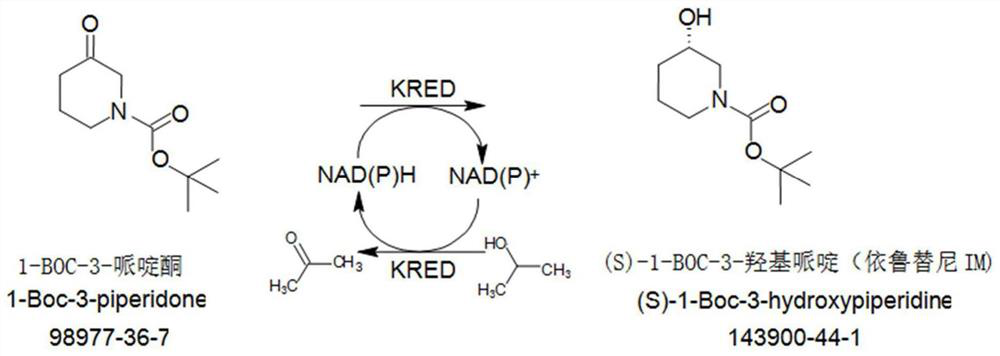
Enzymatic preparation method of ketoreductase and S-1-BOC-3 hydroxypiperidine
A 1-BOC-3, catalytic preparation technology, applied in microorganism-based methods, biochemical equipment and methods, oxidoreductases, etc., can solve the problems of many steps, low substrate concentration, uneconomical and environmental protection, etc. High purity, less coenzyme dosage and cost reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1 Preparation of ketoreductase thallus and enzyme powder
[0029] Inoculate the Escherichia coli strain whose nucleotide sequence is as shown in SEQ ID NO: 1 in the sequence table on LB solid medium containing chloramphenicol resistance, and culture at 37° C. for 20 h. Pick a single colony and inoculate it in 50mL LB liquid medium containing chloramphenicol resistance, shake it for 20 hours, transfer the bacterial liquid to 250mL TB liquid medium after culturing, and take the bacterial liquid to dilute after 2.5 minutes to detect the OD value of 0.7. Add 0.1 mM isopropyl-β-D-thiogalactoside to induce protein expression, shake culture at 30°C for 18 hours, and collect bacteria by centrifugation at 8000 rpm.
[0030] The prepared thallus was lysed back with 0.1mol / L PBS buffer solution (pH=7.0), homogeneously crushed, centrifuged to collect the supernatant of the enzyme solution and freeze-dried to obtain ketoreductase (enzyme powder), wherein, the The added a...
Embodiment 2
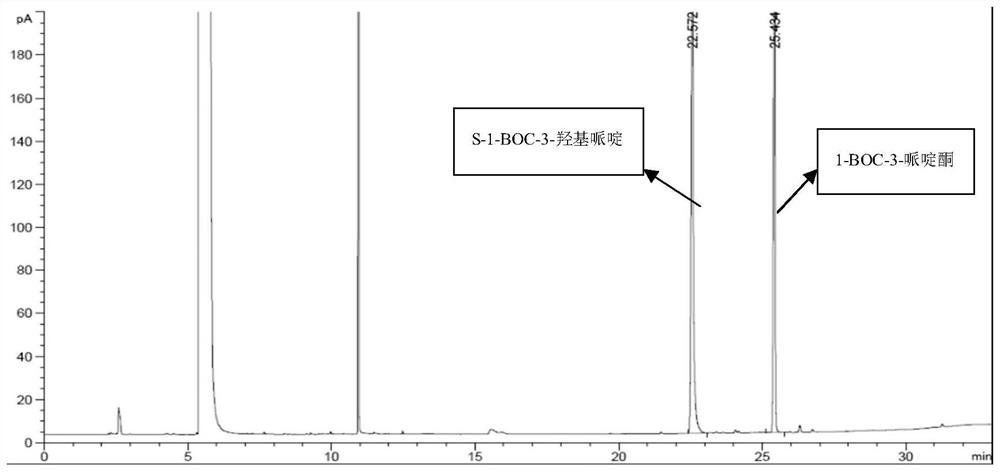
[0031] Example 2 1-Boc-3-piperidone conversion detection method
[0032] GC analysis method: chromatographic column DB-WAX 30m*0.25μm*0.25mm, detector: FID, injection temperature: 230°C, detector temperature: 300°C, flow rate: 1.0mL / min, split ratio: 20:1, Injection volume: 1uL, analysis time 44min, retention time of 1-BOC-3 piperidone is 25.434min, retention time of S-1-BOC-3 hydroxypiperidine is 22.572min.
Embodiment 3
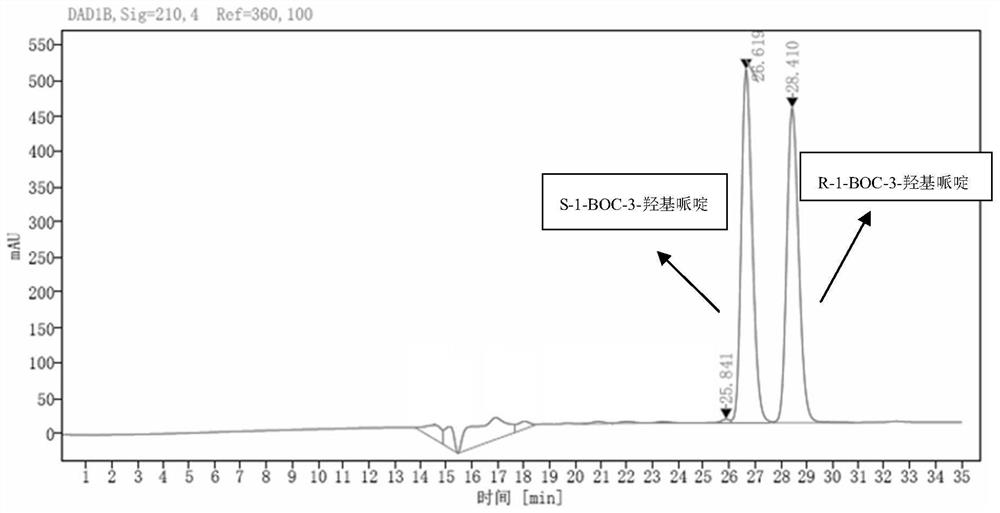
[0033] Example 3 S-1-BOC-3-hydroxypiperidine optical purity detection method
[0034] HPLC analysis method: analysis column CHIRALPAKIG, mobile phase is 0.1% formic acid aqueous solution and methanol, flow rate is 0.2mL / min, column temperature is 25°C, detection wavelength is 210nm, analysis time is 4min, S-1-BOC-3 hydroxypiperidine The retention time of is 26.619min, and the retention time of R-1-BOC-3 hydroxypiperidine is 28.410min.
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com