Cathepsin K inhibitor containing naphthoquinone and phenanthrenequinone structures, composition and application thereof
A technology of cathepsin and inhibitors, which is applied in the field of medicine, can solve the problems of tumor cell apoptosis, achieve the effects of increasing bone density, inhibiting the activity of human osteoclasts, and improving bone structure characteristics and physiological state
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Preliminary Screening of Cathepsin K Inhibitors
[0034] The identification of cathepsin K inhibitors was carried out by examining the ability of compounds to inhibit the binding of cathepsin K to the fluorescently labeled synthetic substrate Z-FR-MCA.
[0035] 1 Experimental materials
[0036] The monomeric compound β-lapachone was purchased from Merck; menatetrenone and menadione heptaquinone were purchased from Sigma; the substrate Z-FR-MCA was purchased from WAKO, Japan.
[0037] 2 Experimental methods
[0038] The inhibitor was diluted with 100mM sodium acetate buffer (pH5.5, containing 2.5mM DTT and 2.5mM EDTA) to a concentration of 25μM, and added to a 96-well plate with a final reaction volume of 200uL. Add final 100, 75, 25, 15, 5μM) CatK with a final concentration of 5nM and incubate for 5 minutes, then add 5μL substrate 1mM Z-FR-MCA solution to start the reaction, and detect the fluorescence signal (divergent light wavelength 460nm, absorption li...
Embodiment 2
[0043] Example 2: Molecular docking of vitamin K2 and cathepsin K
[0044] Use the libdock molecular docking method in Discovery studio 2016 software to investigate the compound and cathepsin K (1ATK) protein for rigid docking.
[0045] 1 Experimental method:
[0046] The LibDock score is used as the core index to characterize the best binding position of the compound. The higher the LibDock score, the higher the predicted activity of the small molecule binding to the receptor. In addition, the number of hydrogen bonds between the ligand and the receptor was analyzed by DS2016 software, and the amino acid residues that made an important contribution to the ligand binding were found.
[0047] 2 Experimental results
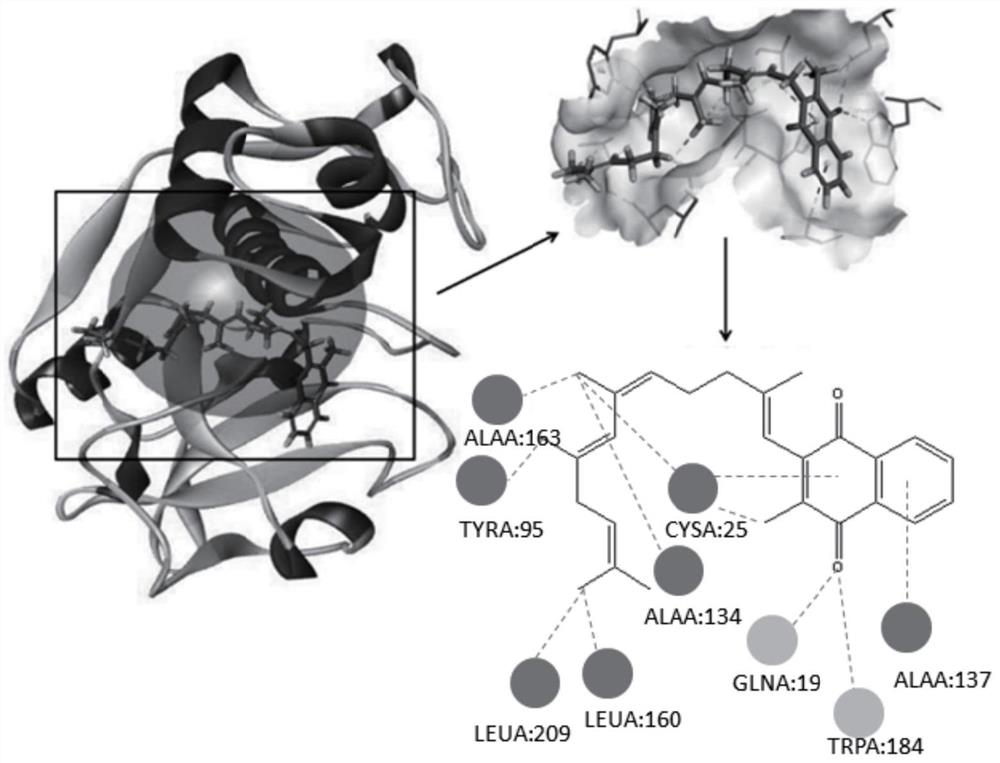
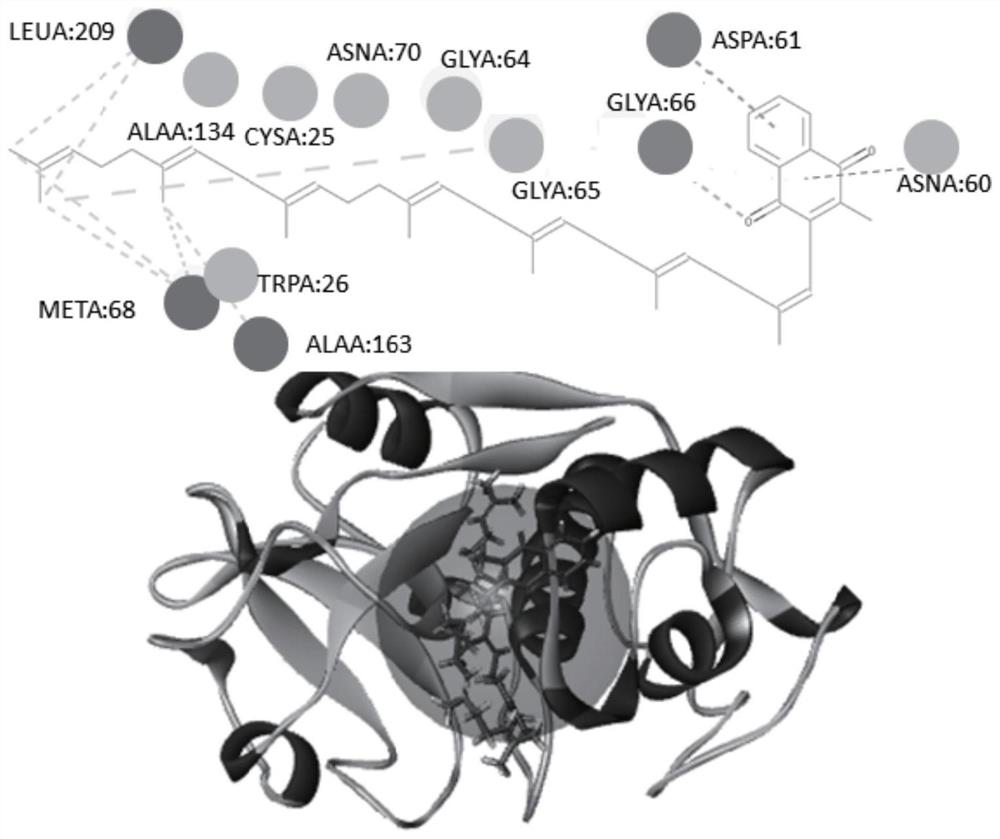
[0048] For libdock molecular docking in Discovery studio 2016 software, vitamin K2 docking scores were 128 for MK4 and 119 for MK7, reflecting a strong docking effect.
[0049] by two-dimensional plane figure 1 It can be seen that there are obvious hydrogen bo...
Embodiment 3
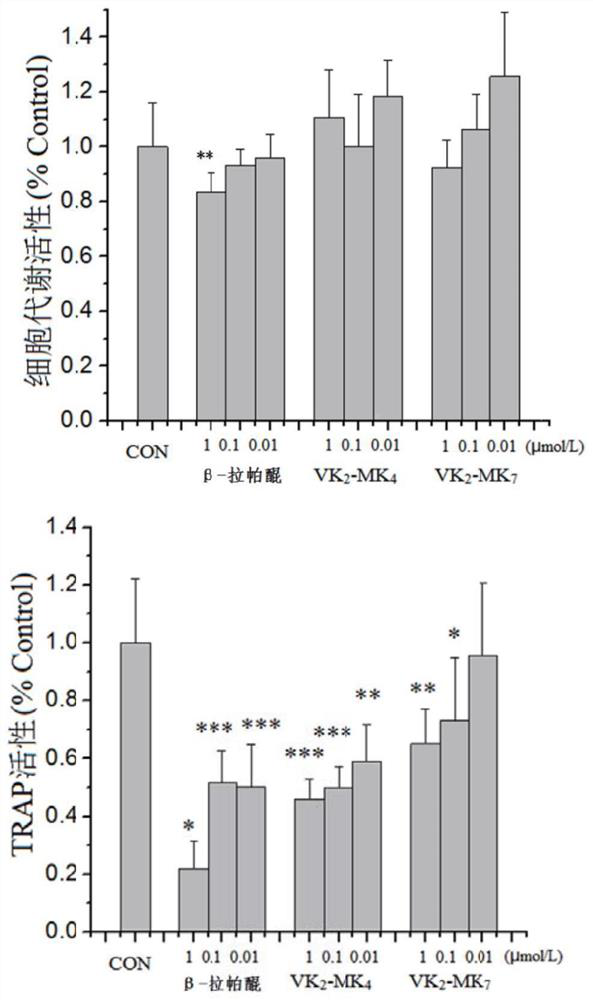
[0051] Example 3: Influence of Inhibitors on the Activity of Cathepsin K
[0052] Cathepsin K inhibitors inhibit cathepsin K activity primarily through its ability to degrade the activity of natural substrates collagen fibers, elastic fibers, and thyroglobulin.
[0053] 1 Experimental materials
[0054] Monomer compound β-lapachone was purchased from merck company; Menadione tetraenyl and menadione heptaquinone were purchased from Sigma company; Chondroitin sulfate A (C4-S), E-64 and pepsin were purchased from Sigma, USA company; type I collagen was purchased from Affymetrix Company of the United States; microporous filter membrane centrifuge tubes were purchased from Amico Millipore Company.
[0055] 2 Experimental methods
[0056] 2.1 Collagen fiber degradation activity
[0057] Using gel electrophoresis (SDS-PAGE), soluble type I collagen was dissolved in 100mM sodium acetate buffer (pH 5.5, containing 2.5mM DTT and 2.5mM EDTA) to a final concentration of 0.6mg / ml, follo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com