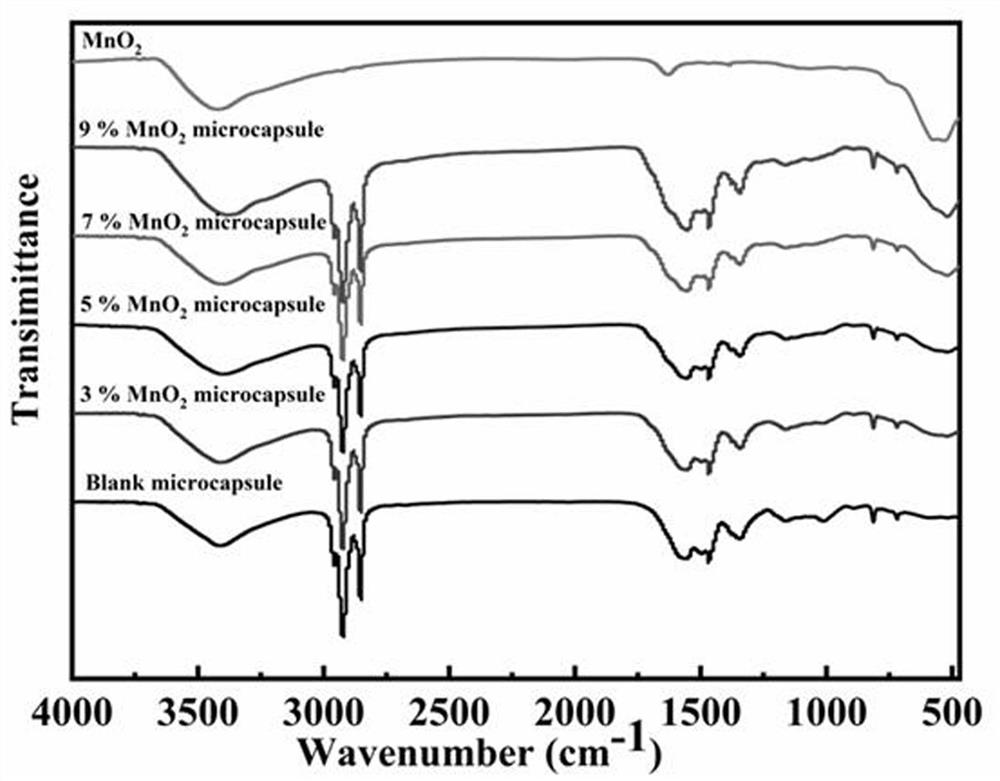
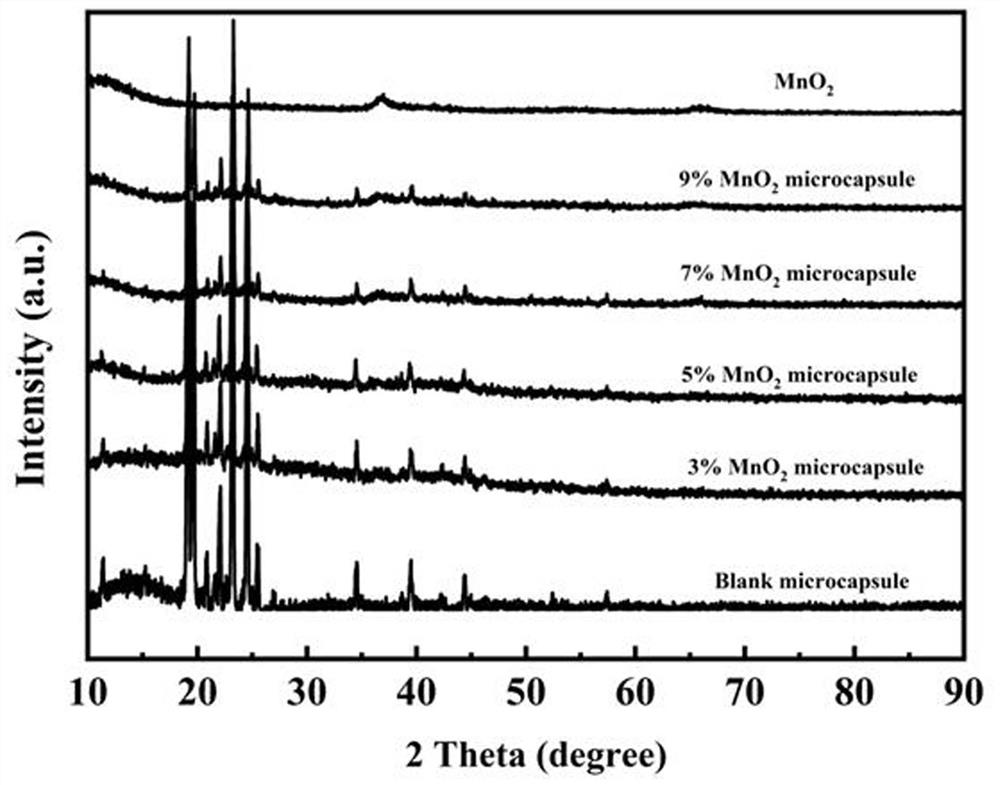
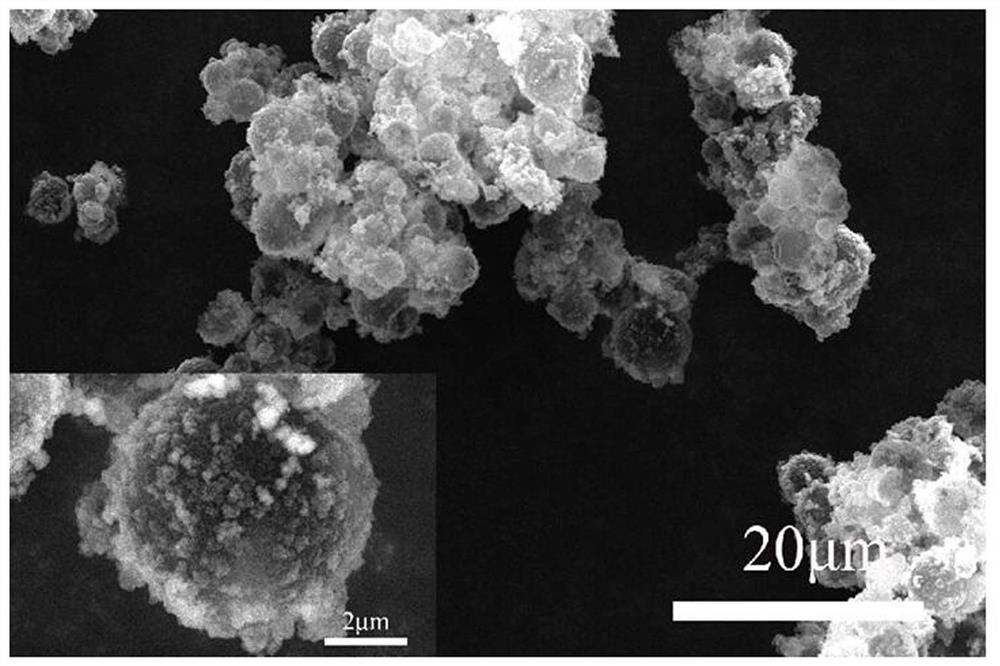
Manganese dioxide-melamine formaldehyde resin double-shell composite phase change material and preparation method thereof
A composite phase change material, melamine technology, applied in chemical instruments and methods, manganese oxide/manganese hydroxide, microsphere preparation, etc., can solve problems such as easy aggregation of nanoparticles, poor photothermal performance, and difficulty in achieving additive effects. Achieve high photothermal performance, maintain thermal stability, and achieve small changes in phase transition temperature and phase transition latent heat
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] A kind of preparation method of manganese dioxide-melamine formaldehyde resin double-shell composite phase change material:
[0056] Step 1) Pretreatment of raw materials, weigh 6g of melamine, 10ml of formaldehyde, and 10ml of deionized water in a single-necked flask, stir and mix evenly at room temperature to obtain solution A, and then dropwise add triethanolamine solution with a volume fraction of 50% to adjust the pH value to 9, and then stirred at 70°C for 1 hour for prepolymerization to obtain a prepolymer, and another 25 g of n-octadecane and 7.5 g of styrene maleic anhydride, wherein the styrene maleic anhydride was first prepared as mass A styrene maleic anhydride solution with a fraction of 5% was emulsified under the conditions of a rotation speed of 2500r / min, an emulsification temperature of 70, and an emulsification time of 2 hours to obtain an emulsion;
[0057] Step 2) Preparation of microcapsules. Under the conditions of temperature 70°C and rotational...
Embodiment 2
[0079] A method for preparing a manganese dioxide-melamine formaldehyde resin double-shell composite phase change material, the steps not specifically described are the same as in Example 1, except that the quality of manganese sulfate in the step 3 is 0.065g, 0.0167 The volume of g / mL potassium permanganate is 15ml, and the resulting sample is named 3wt% MnO 2 microcapsule.
[0080] In order to prove the phase change properties of manganese dioxide-melamine formaldehyde resin double-shell composite phase change materials, differential scanning calorimetry (DSC) tests were carried out. The result is as Figure 4 As shown, the melting temperature of the manganese dioxide-melamine formaldehyde resin double-shell composite phase change material is 29.70°C, and the crystallization temperature is 23.50°C; the measured latent heat of phase change is 152.71J / g and 151.74J / g.
[0081] In order to prove the photothermal conversion effect of manganese dioxide-melamine formaldehyde re...
Embodiment 3
[0083] A method for preparing a manganese dioxide-melamine formaldehyde resin double-shell composite phase change material, the steps not specifically described are the same as in Example 1, except that the quality of manganese sulfate in the step 3 is 0.10g, 0.0167 The volume of g / mL potassium permanganate is 25ml, and the resulting sample is named 5wt% MnO 2 microcapsule.
[0084] In order to prove the phase change properties of manganese dioxide-melamine formaldehyde resin double-shell composite phase change materials, differential scanning calorimetry (DSC) tests were carried out. The result is as Figure 4 As shown, the melting temperature of the manganese dioxide-melamine formaldehyde resin double-shell composite phase change material is 29.64°C, and the crystallization temperature is 23.61°C; the measured latent heat of phase change is 142.13J / g and 141.44J / g.
[0085] In order to prove the photothermal conversion effect of manganese dioxide-melamine formaldehyde res...
PUM
| Property | Measurement | Unit |
|---|---|---|
| phase transition temperature | aaaaa | aaaaa |
| phase transition enthalpy | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com