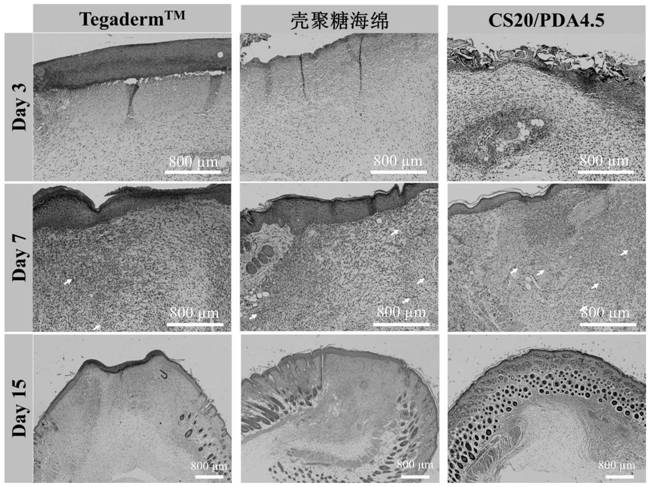
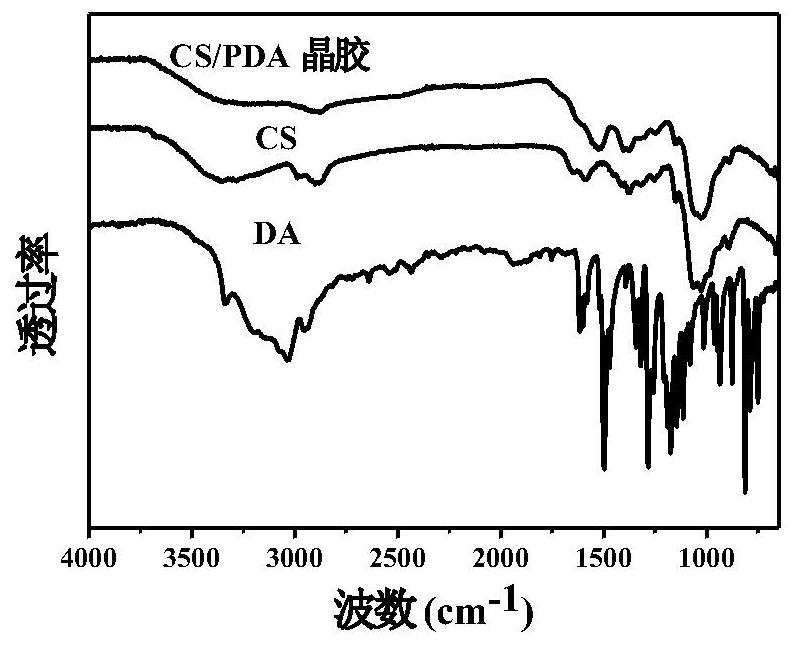
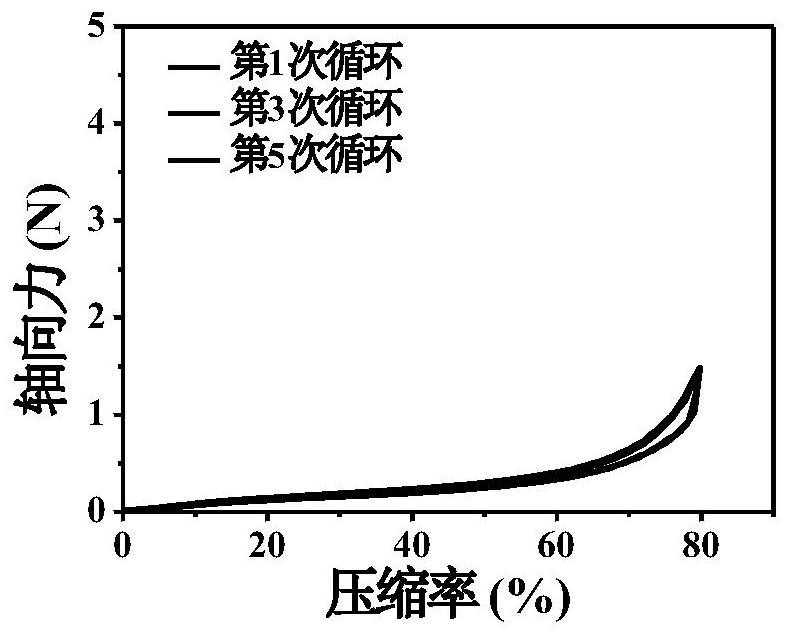
An injectable degradable dry hemostatic gel with good shape memory and blood coagulation ability, its preparation method and application
A technology of blood coagulation and crystal gel, applied in the field of biomedical materials, to achieve the effect of accelerating blood coagulation and promoting the formation of blood vessels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] The preparation method of the present invention comprises the following steps:
[0045] 1) Resuspend chitosan (CS) in deionized water, then add glacial acetic acid dropwise with stirring, and then heat and stir at 50-60°C for 30-60 minutes to obtain CS solution; in CS solution, chitosan, deionized water The ratio between glacial acetic acid and glacial acetic acid is 1.0g:(39~160)mL:(1000~4000)μL; add dopamine hydrochloride (DA) into deionized water to make DA solution;
[0046] 2) After the CS solution, DA solution and oxidant solution are pre-cooled in an ice bath, mix the CS solution and DA solution, and at the same time add the oxidant solution and mix evenly to obtain a mixed solution, wherein the final mass concentration of CS is 0.5~ 2.0%, the final concentration of DA is 0.5-9 mg / mL; the oxidizing agent is sodium periodate; the molar ratio of sodium periodate to dopamine is 0.5-1:1;
[0047] 3) Put the mixed solution at -7~-20°C for 12~36h to obtain the frozen ...
Embodiment 1
[0052] Preparation of CS20 / PDA0.5 crystal gel: resuspend chitosan in deionized water, then add glacial acetic acid dropwise with stirring, and then heat and stir at 55°C for 40min to obtain 2.5wt% CS solution. In CS solution, chitosan The ratio between sugar, deionized water and glacial acetic acid is 1.0g: 39mL: 1000μL; add dopamine hydrochloride to deionized water to make a 5mg / mL DA solution; add sodium periodate to deionized water to make a 5.64 mg / mL SP solution; the molar ratio of sodium periodate to dopamine is 1:1. Subsequently, the CS solution, the DA solution and the SP solution were fully pre-cooled in an ice-water mixing bath and then fully mixed to obtain a final concentration of CS of 2.0 wt % and a final concentration of DA of 0.5 mg / mL. Then the mixture was placed in a low-temperature reactor at -20°C for 36 h. After the reaction, the crystal gel was melted in deionized water and freeze-dried at -80°C to obtain the injectable and degradable dry crystal gel hem...
Embodiment 2
[0054] The final concentration of DA in the step was controlled at 1.5 mg / mL, and other conditions were the same as in Example 1 to obtain CS20 / PDA1.5 crystal gel.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com