Application of pregnancy test paper to on-site instant detection of hepatitis B virus drug-resistant mutant genes
A hepatitis B virus and drug-resistant mutation technology, which is applied in the direction of resistance to vector-borne diseases, microbial measurement/inspection, biochemical equipment and methods, etc., can solve the problem of gene chip method being expensive, complicated steps, time-consuming, etc. problem, to achieve the effect of specific and portable detection and intuitive results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
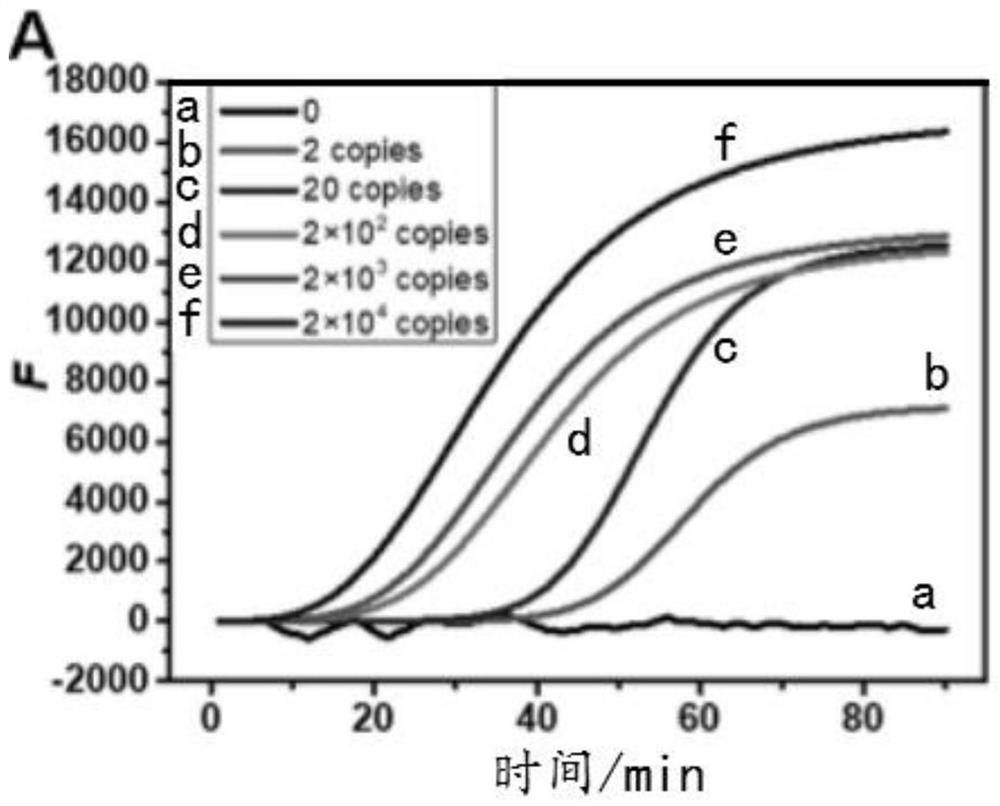
[0070] Example 1 Real-time detection of HBV drug-resistant mutant genes by LAMP-coupled strand-displacement probes to verify the effectiveness of LAMP amplification primers
[0071] experimental method:
[0072] 1. Prepare 25 μL reaction system, including external primer pair (SEQ ID NO:1 and SEQ ID NO:2), internal primer pair (SEQ ID NO:3 and SEQ ID NO:4), dNTP, betaine and buffer, The buffer is 1× isothermal amplification buffer, add 1 μL concentration of 0, 2copies / μL, 20copies / μL, 2×10 2 copies / μL, 2×10 3 copies / μL, 2×10 4 Copies / μL HBV mutant template (SEQ ID NO:5); after annealing, add fluorescent quenching strand displacement probes (SEQ ID NO:7 and SEQ ID NO:8), Bst 2.0 DNA polymerase; finally put into real-time In a fluorescent quantitative PCR instrument, react at 63°C for 90min.
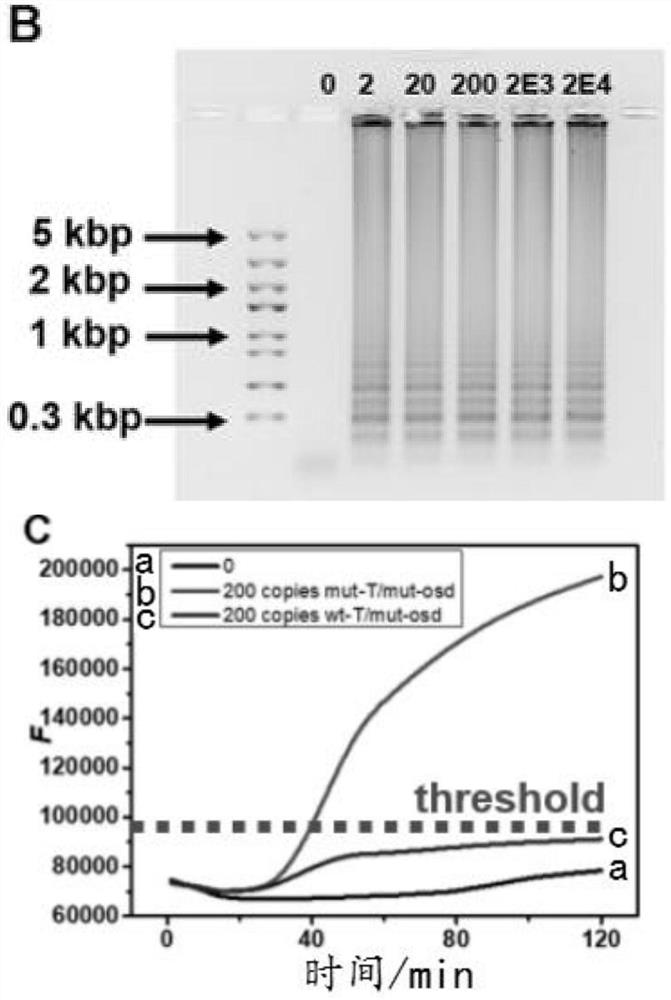
[0073] 2. The LAMP amplification product was characterized by 1% agarose gel electrophoresis. Weigh 0.25g agarose and dissolve it in 25mL 1×TAE solution, heat in microwave until disso...
Embodiment 2 3
[0078] Example 2 Preparation and Stability Test of Three-Dimensional Large Volume DNA Molecules
[0079] experimental method:
[0080] 1. Prepare a 20 μL reaction solution system, including RCA template (SEQ ID NO:12), RCA primer (SEQ ID NO:13), T4 DNA ligase and buffer, the buffer is 1×DNA ligase buffer, react at room temperature 3h.
[0081] 2. Prepare a 20 μL reaction solution system, including phi29 DNA polymerase, dNTP, BSA and buffer, the buffer is 1×RCA amplification buffer, and mix with the above reaction solution, react at 30°C for 48 hours, and react at 75°C for 10 minutes , washed by centrifugation, and redispersed in 40 μL HO 2 O middle.
[0082] 3. Vacuum freeze-dry the reaction product, and disperse it in water after being stored at room temperature for different periods of time, and investigate its morphology change.
[0083] Experimental results:
[0084] Such as image 3 As shown, there is no obvious shape change after lyophilization and storage at room ...
Embodiment 3
[0085] Example 3 Concentration Gradient Detection of Pregnancy Test Paper on HBV Drug Resistance Mutant Gene LAMP Amplified Simulation Loop Sequence
[0086] experimental method:
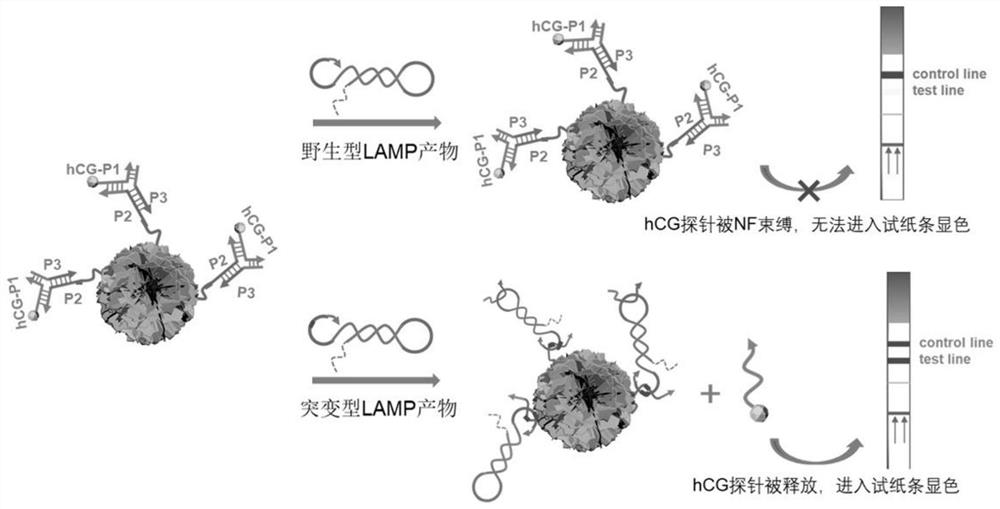
[0087]1. Coupling hCG signaling molecules on probe P1 as a signal probe; hybridizing probe P1, probe P2 and probe P3 to generate a three-way probe; wherein, probe P2 and probe P3 are used as detection probes .
[0088] 2. Configure a 20 μL system, including 5 μL of the rolling circle amplification product (the three-dimensional large-volume DNA molecule obtained in Example 2) and 20 nM three-way probe, and react at room temperature for 1 h.
[0089] 3. Add 0, 0.5nM, 2nM, 5nM, 20nM, 50nM and other different concentrations of HBV drug-resistant mutant gene LAMP amplification simulation loop sequence (synthesized by Sangon Bioengineering (Shanghai) Co., Ltd., the HBV The sequence of the simulated circle amplified by LAMP of the drug-resistant mutant gene is the same as that of the single-stranded DNA...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com