Purification preparation method of ganglioside monomers
A ganglioside and monomer technology, applied in chemical instruments and methods, preparation of sugar derivatives, organic chemistry, etc., can solve the problems of sample loss, high organic residues, different charges, etc. High sample load and good resolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] The flow process of a purification preparation method of a ganglioside monomer of the present invention comprises the following steps:
[0022] Step 101 dissolving the sample: dissolving the pretreated ganglioside graded product with water and an organic solvent at a ratio of 90 / 10 to 10 / 90 to obtain a solution;
[0023] Here, the pretreated ganglioside fraction product contains one or more ganglioside monomers in GA, GM, GD, GT, and GQ;
[0024] Preferably, the organic solvent is one or more of isopropanol, methanol, ethanol, acetonitrile, n-butanol, methylene chloride, chloroform, ethylene dichloride, tetrahydrofuran;
[0025] In the sample dissolving step described here, the solution can be obtained by ultrasonically dissolving and passing through a 0.45 μm organic film;
[0026] Step 102 Configure the mobile phase: use the organic solvent-buffered saline solution as the mobile phase, wherein the volume concentration of the organic solvent is 5%-95%, the concentrati...
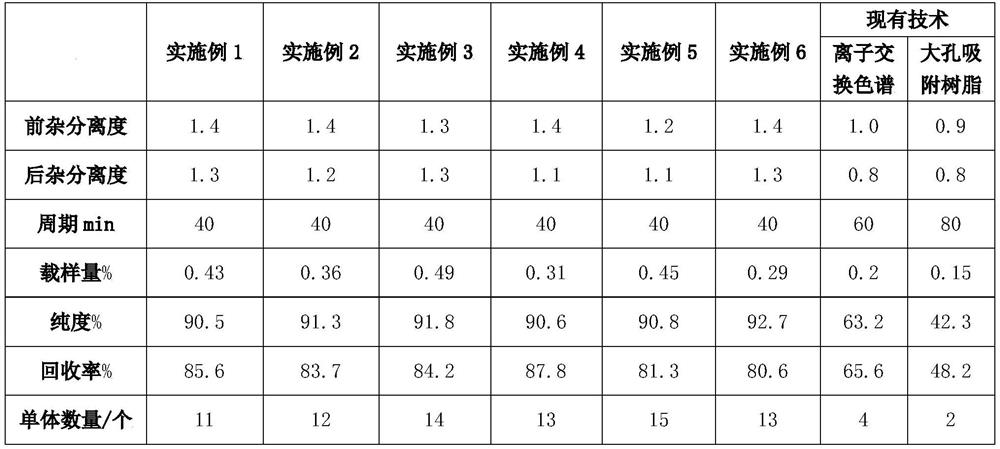
Embodiment 1
[0037] Step 1: Take the graded product of gangliosides in a test tube, add 50% methanol to prepare a 50 mg / ml sample, dissolve it with ultrasonic waves, and pass through a 0.45 μm organic membrane to obtain a clear solution;
[0038] Step 2: Use a C8 reverse-phase chromatographic column with a column size of 4.6×250mm, a particle size of 5μm, and a pore size of The injection volume was 13 mg, the flow rate was 0.7 mL / min, the column temperature was 35 °C, the detection wavelength was 205 nm, the mobile phase was acetonitrile and 100 mM ammonium dihydrogen phosphate (pH=6.50), and the concentration of acetonitrile ranged from 45 to 40 min in 0 to 40 min. %~60%, gradient elution, collected according to peak shape;
[0039] Step 3: Collect the eluted samples and carry out rotary evaporation and concentration treatment at 40°C at a rotation speed of 70r / min to obtain the separated ganglioside monomers.
[0040] The separated gangliosides were identified by mass spectrometry. The...
Embodiment 2
[0042]Step 1: Take the graded product of gangliosides in a test tube, add 50% methanol to make a 100 mg / ml sample, dissolve it by ultrasonic, pass through a 0.45 μm organic membrane to obtain a clear solution;
[0043] Step 2: Use a C8HC reverse-phase chromatographic column with a column size of 10×250mm, a particle size of 10μm, and a pore size of The injection volume is 54mg, the flow rate is 2mL / min, the column temperature is 35°C, the detection wavelength is 205nm, the mobile phase is acetonitrile, 50mM diammonium hydrogen phosphate (pH=6.50), and the concentration of acetonitrile within 0-40min is from 45%~60%, gradient elution, collected according to peak shape;
[0044] Step 3: Collect the eluted samples and carry out rotary evaporation and concentration treatment at 40°C at a rotation speed of 70r / min to obtain the separated ganglioside monomers.
[0045] The separated gangliosides were identified by mass spectrometry. The average purity of ganglioside monomers can r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com