Mycoplasma synoviae culture medium and preparation method thereof
A technology of mycoplasma synovialum and culture medium, which is applied in the field of mycoplasma synovialum culture medium and its preparation, can solve problems such as unfavorable diagnosis and clinical drug screening, affecting the protective efficacy of finished vaccines, and heavy concentration operations of semi-finished products, and achieves improved The effect of harvesting the total amount of antigen, improving product stability, and increasing the rate of material transfer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] A culture medium for Mycoplasma gallisanum bursa, comprising basal medium and auxiliary components, the basal medium includes phosphate buffer, glucose, hydrolyzed milk protein, coenzyme I, arginine hydrochloride, MEM, yeast extract powder, 1 % phenol red and trehalose; auxiliary components include porcine serum and penicillin.
[0024] The weight of each component in the above basal medium is: 800-1000ml of phosphate buffer, 5-18g of glucose, 3-9g of hydrolyzed milk protein, 0.5-1.5g of coenzyme I, 5-15ml of arginine hydrochloride, L-cysteine hydrochloride 0.5 ~ 1.5g, MEM 2 ~ 5g, yeast extract powder 1-5g, 1% phenol red, trehalose 1-5g; the concentration of each component in the above auxiliary components is: pig serum 5 -10% and 800,000 units / ml penicillin 1-2ml / L.
[0025] As an implementation of this example, in the culture medium: 900ml of phosphate buffer, 15g of glucose, 6g of hydrolyzed milk protein, 1g of coenzyme I, 10ml of arginine hydrochloride, 1g of L-c...
Embodiment 2
[0034] Take the chickens with obvious joint swelling in the clinically affected chicken flock, a total of 5 chicken farms, 6 chickens in each chicken farm. Separation is carried out by the MS medium, the improved Frey medium, and the improved CM medium in Example 1 of the present invention, respectively.
[0035] Take the trachea / cleft palate swab and joint fluid of each chicken and dissolve them in 5ml of MS medium, shake and mix well, use a 0.45um filter to sterilize, take 1ml of each and inoculate into the three kinds of medium, and let it stand at 37°C After 2 days of culture, if the color does not change, the first generation is blindly passed according to 10% of the inoculum size; if the second generation of blind passage does not change color after 10 days, it is negative; if the color of the medium changes, it is identified by PCR and sent for sequencing identification.
[0036] The separation results of the sensitivity of the three media to MS are as follows:
[0037...
Embodiment 3
[0040] 1. Culture of Mycoplasma gallinarum bursa
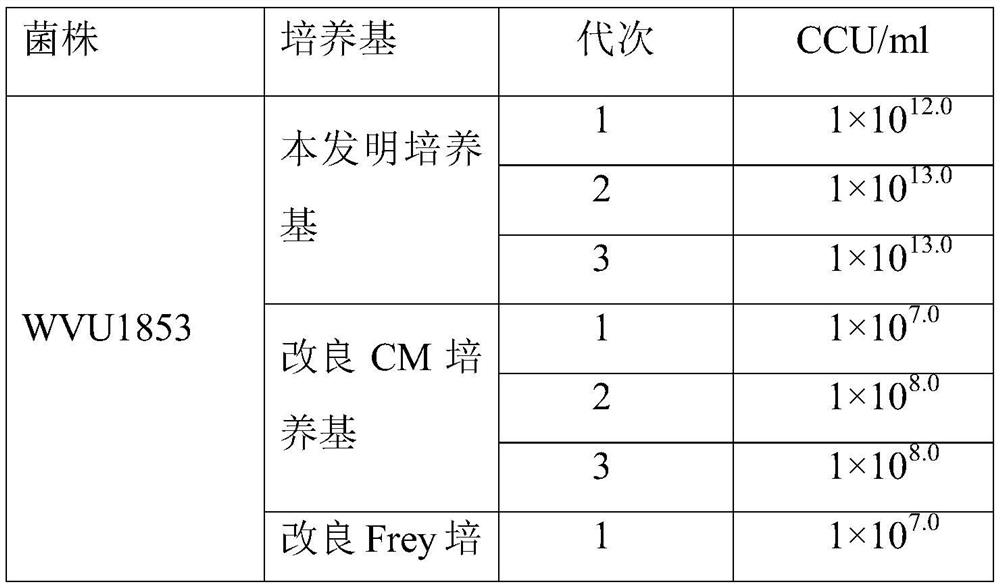
[0041] The culture medium selected in this experiment is Mycoplasma gallinarum WVU1853 strain, which was isolated and preserved clinically. Z1 seeds were inoculated in the improved CM medium, the improved Frey medium, and the MS medium of Example 1 according to the volume ratio (V:V) of 10%. After fully mixing, they were shaken and cultured at 100r / min at 37°C. When the color of the culture solution Turn yellow and harvest when the pH drops from 7.6 to 6.8. Passage for 3 generations in the same way, record the passage time and measure the discoloration unit and protein concentration of the 3rd generation.
[0042] 2. Viable bacterial titer (CCU) determination method
[0043]Take 28 small test tubes for each sample (outer diameter (mm) × length (mm) is 12:100), each tube is filled with 1.8ml of culture medium, and 0.2ml of the culture that needs to measure the titer of viable bacteria is added to the first test tube Mix the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com