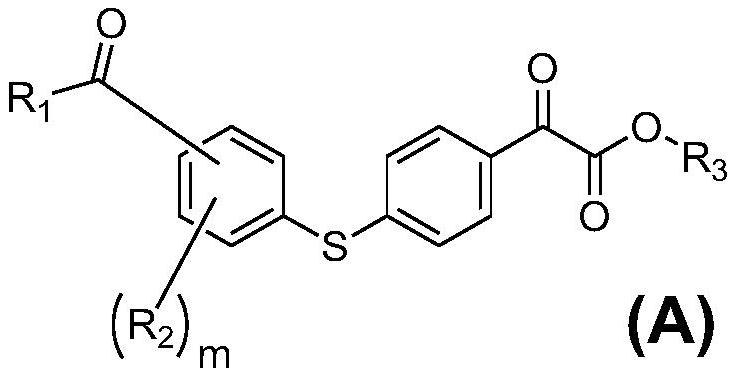
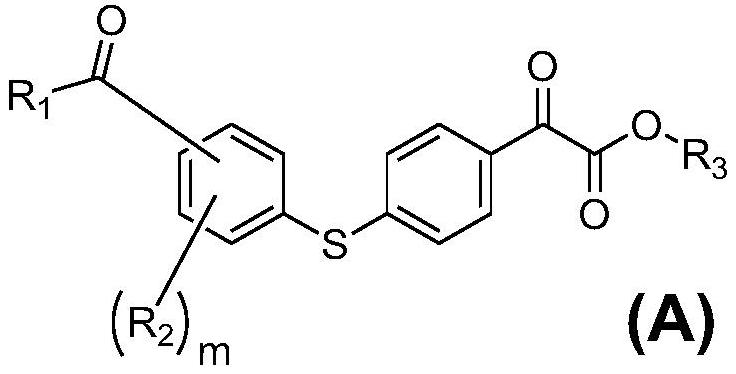
Water-soluble photopolymerization initiator containing diphenyl sulfide ketone formate and preparation method of water-soluble photopolymerization initiator
A diphenyl sulfide ketoformic acid and photoinitiator technology, which is applied in the field of diphenyl sulfide ketoformate photoinitiators, can solve the problems of limited use, achieve good water solubility, convenient purification, and simple synthesis method Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: the synthesis of (A)-1 to (A)-18
[0026] The preparation process of compound (A)-1
[0027]
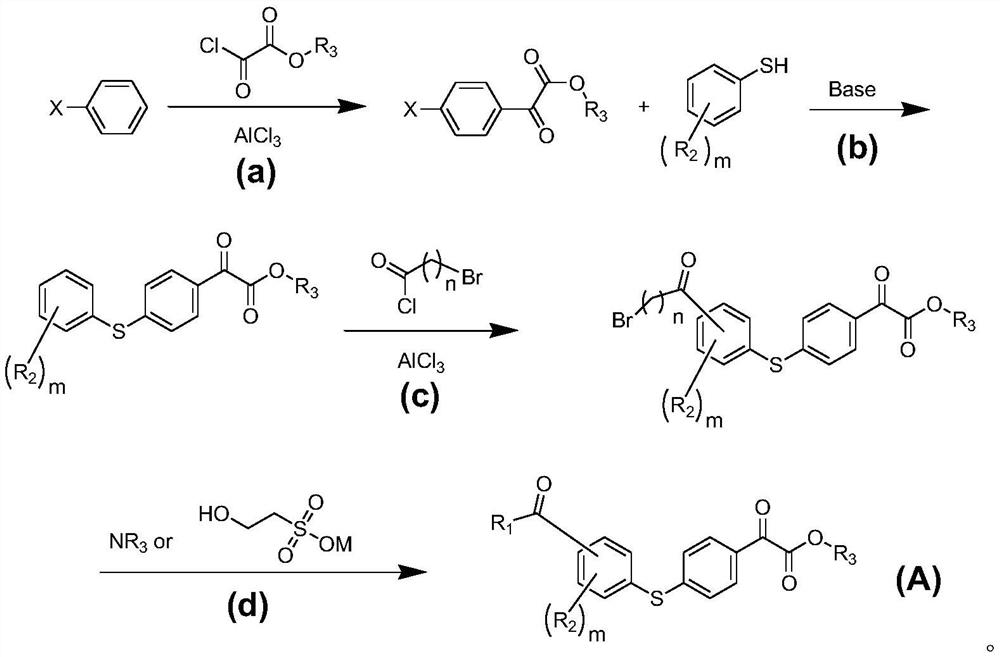
[0028] (a) Dissolve bromobenzene (0.1mol) in 100 ml of anhydrous dichloromethane, add anhydrous aluminum chloride (0.2mol) in batches, add monomethyl oxalyl chloride (0.1mol) dropwise at 0-10°C , the dropwise addition is completed in half an hour, the reaction is stirred at room temperature for 2-6 hours, the reaction is completed by pointing the plate, slowly added to ice water equal to the volume of the solvent, the organic layer is washed with deionized water, dried with anhydrous sodium sulfate, and can be prepared by distillation under reduced pressure Methyl 4-bromophenyl ketone formate was directly used in the next reaction.
[0029] (b) Methyl 4-bromophenyl ketone formate (0.05mol) and thiophenol (0.05mol) were dissolved in N, N-dimethylformamide (DMF) (100 ml), potassium carbonate (0.06 mol) and CuI (0.006mol), stirred and reacted at 140°C for 4 hou...
Embodiment 2
[0051] Embodiment 2: Synthesis of (A)-19 to (A)-24
[0052] The structural formula of compound (A)-19
[0053]
[0054] Add dimethyl sulfoxide (100 ml) and sodium 2-hydroxyethanesulfonate (0.05 mol) to (A)-1c (0.05 mol) obtained by the same operation as above, and stir the reaction at 90°C for 24 hours, and monitor the reaction by spot plate After the reaction was completed, 200 ml of saturated saline was added to the reaction solution, extracted three times with 200 ml of acetonitrile, dried over magnesium sulfate, concentrated under reduced pressure to obtain a light yellow solid, which was recrystallized with isopropanol to obtain (A)-19 , 90% yield.
[0055] (A)-20 was synthesize|combined by the same reaction except having changed sodium 2-hydroxyethanesulfonate into potassium 2-hydroxyethanesulfonate.
[0056] (A)-21 and (A)-22 were synthesized by the same reaction as (A)-20 and (A)-21, respectively, except that monoethyl oxalyl chloride was replaced with monomethyl ...
Embodiment 3
[0057] Embodiment 3: the synthesis of (A)-23
[0058]
[0059] (a) methyl 4-bromophenyl ketone formate (0.05mol) and 2,4,6-trimethylthiophenol (0.05mol) were dissolved in 100 ml of DMF, anhydrous potassium carbonate (0.06mol) was added and CuI (0.006mol), under the protection of nitrogen, stirred at 100°C for 4 h, spotted the plate to monitor the reaction, recovered DMF under reduced pressure, added the residue to 100 ml of water, and extracted the two parts with 100 ml of 1,2-dichloroethane. After drying with anhydrous sodium sulfate, concentrate and recrystallize with ethanol, the product is light yellow powder (A)-7b with a yield of 85%.
[0060] (b) (A)-7b (0.04mol) was added to 50 ml of anhydrous dichloromethane, anhydrous aluminum chloride (0.12mol) was added in portions, and chloroacetyl chloride was added dropwise at 0-10°C ( 0.04mo) of dichloromethane solution, and stirred at room temperature for 10 hours, point plate monitoring after the end of the reaction. The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com