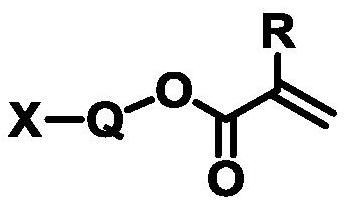
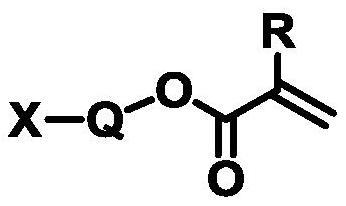
Cycloalkane-based ultraviolet curing packaging ink as well as use method and application thereof
A technology of cycloalkane and ultraviolet light, applied in ink, application, photovoltaic power generation, etc., can solve problems such as unsatisfactory visible light transmittance at 400-780 nanometers, affecting normal display and lighting of devices, and reducing photoelectric conversion efficiency of devices. Achieve the effects of reducing preparation time, accelerating cross-linking rate, and improving aggregation behavior
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] The preparation method of UV-curable composition ink is specifically as follows:
[0044] The cycloalkane monomer containing substituents, the ultraviolet curable monomer and the photocrosslinking initiator are respectively 5% by weight to 85% by weight, 5% by weight to 85% by weight, and 0.1% by weight to 10% by weight Store in a stainless steel container, stir and mix at room temperature in the dark until the photocrosslinking initiator dissolves.
[0045] The usage method of UV curable composition ink is as follows:
[0046] By one of the methods such as spin coating, scraping coating or inkjet printing, the prepared ink is evenly attached to the surface of the electronic device to be packaged, and then the ink is cured by ultraviolet light to obtain an organic film. The present invention adopts the method of inkjet printing, prints the ink of the UV curable composition into a liquid film of a certain shape, and then adjusts the power of the UV lamp at 10-500mW / cm ...
Embodiment 1
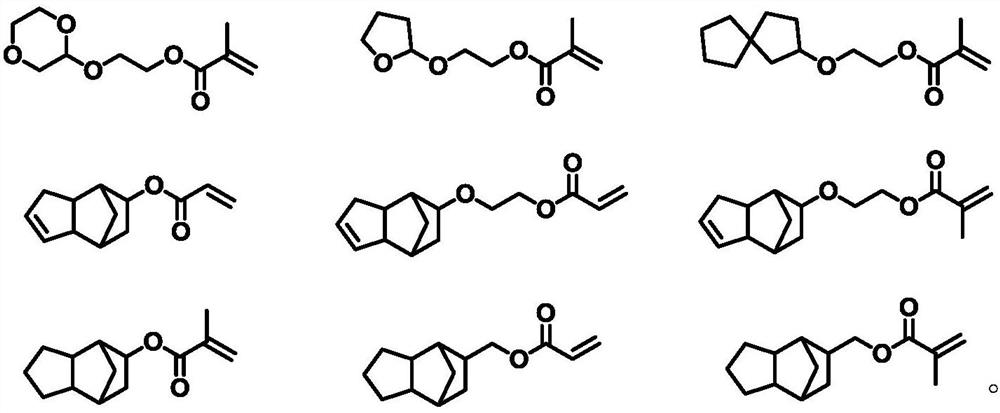
[0050] Synthesis of 2-((1,4-dioxan-2-yl)oxy)ethyl methacrylate
[0051]
[0052] In a nitrogen atmosphere, add 16.7 g of 2-bromo-1,4-dioxane, 6.2 g of ethylene glycol, 27.6 g of potassium carbonate, and 0.26 g of copper chloride into the reaction flask, heat to 150°C and stir for 48 hours. The reaction mixture was cooled to room temperature, washed with water and extracted with dichloromethane to obtain an organic layer. The organic layer was dried with anhydrous magnesium sulfate, concentrated to remove dichloromethane, and the residue was purified with a silica gel column to obtain 11.5 g of 2-((1,4-dioxan-2-yl)oxy)ethan-1-ol; Then the resulting 2-((1,4-dioxan-2-yl)oxy)ethan-1-ol and 9.8 g of methacryloyl chloride were dissolved in 200 ml of dry pyridine, stirred at room temperature for 48 hours in a nitrogen atmosphere, and then concentrated , the residue was purified with a silica gel column to obtain 8.5 g of 2-((1,4-dioxan-2-yl)oxy)ethyl methacrylate with a yield of ...
Embodiment 2
[0056] Synthesis of 2-((tetrahydrofuran-2-yl)oxy)ethyl methacrylate
[0057]
[0058] In a nitrogen atmosphere, add 15.1 grams of 2-bromotetrahydrofuran, 6.2 grams of ethylene glycol, 27.6 grams of potassium carbonate and 0.26 grams of copper chloride to the reaction flask, heat to 150 ° C and stir for 48 hours, then cool the reaction mixture to room temperature, wash with water and use An organic layer was obtained by extraction with dichloromethane. Concentrate and remove methylene chloride after drying the organic layer with anhydrous magnesium sulfate, and the residue is purified with a silica gel column to obtain 8.8 grams of 2-((tetrahydrofuran-2-yl)oxy)ethan-1-alcohol; then the resulting 2- ((Tetrahydrofuran-2-yl)oxy)ethan-1-ol and 9.8 g of methacryloyl chloride were dissolved in 200 ml of dry pyridine, stirred at room temperature in a nitrogen atmosphere for 48 hours, concentrated, and the residue was purified with a silica gel column to obtain 6.7 gram of 2-((tetr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Surface tension | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com