Synthesis method of sulfadiazine
A technology of sulfadiazine and its synthesis method, which is applied in the field of synthesis of sulfadiazine, and can solve the problems of high pressure on environmental protection, pollution, and high price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
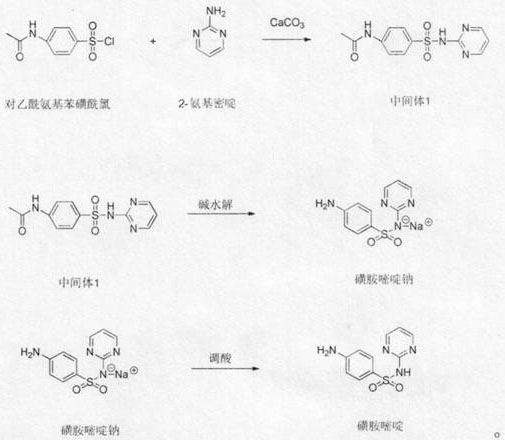
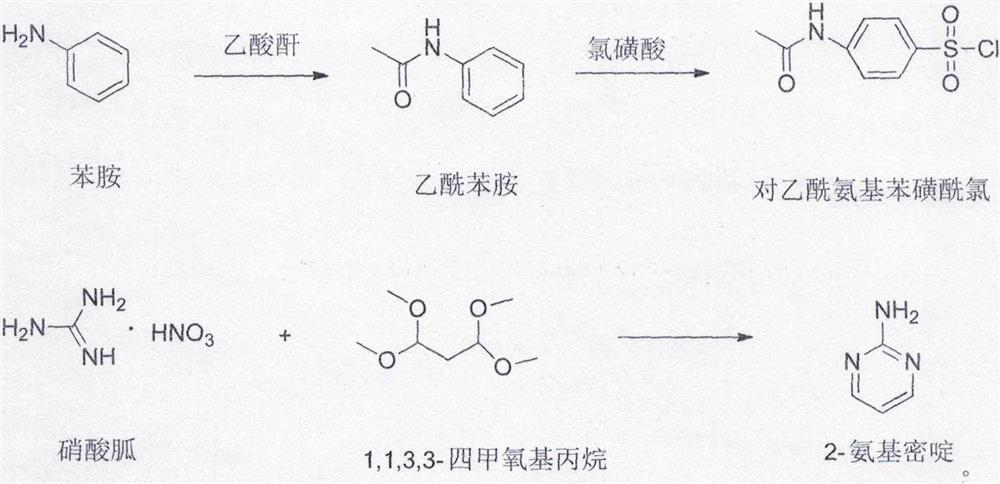
[0018] Add 800g of toluene, 233.7g (1mol) of p-acetamidobenzenesulfonyl chloride, and 95.1 (1mol) g of 2-aminopyrimidine into a 2L reaction flask, stir at 20°C to 30°C, add 200g (2mol) of powdered calcium carbonate, and heat up to 55 ℃ ~ 65 ℃ heat preservation reaction, after the reaction is completed, add 300g of 20% sodium hydroxide aqueous solution, heat up to 100 ℃ ~ 115 ℃ for reaction, after the reaction is completed, cool down, let it stand, the upper layer of toluene layer is used mechanically, and the lower layer of water layer is slowly dripped with concentrated hydrochloric acid, Adjust the pH of the system to 5.0-5.5, filter, wash the filter cake with a large amount of water, and dry to obtain sulfadiazine with a yield of 98.2%.
Embodiment 2
[0020] Add 800g of toluene, 233.7g (1mol) of p-acetamidobenzenesulfonyl chloride, 95.1g (1mol) of 2-aminopyrimidine into a 2L reaction flask, stir at 20°C to 30°C, add 100g (1mol) of powdered calcium carbonate, and heat up to 55 ℃ ~ 65 ℃ heat preservation reaction, after the reaction is completed, add 300g of 20% sodium hydroxide aqueous solution, raise the temperature to 100 ℃ ~ 115 ℃ for reaction, after the reaction is completed, cool down, let it stand, apply the upper layer of toluene, and slowly add concentrated hydrochloric acid dropwise to the lower layer to adjust the system pH 5.0-5.5, filter, wash the filter cake with a large amount of water, and dry to obtain sulfadiazine with a yield of 87.4%.
Embodiment 3
[0022] Add 800g of toluene, 233.7g (1mol) of p-acetamidobenzenesulfonyl chloride, and 95.1g (1mol) of 2-aminopyrimidine in a 2L reaction flask, stir at 20°C to 30°C, add 150g (1.5mol) of powdered calcium carbonate, and heat up to 55 ℃ ~ 65 ℃ heat preservation reaction, after the reaction is completed, add 300g of 20% sodium hydroxide aqueous solution, heat up to 100 ℃ ~ 115 ℃ for reaction, after the reaction is completed, cool down and let it stand, the upper layer of toluene layer is used mechanically, and the lower layer of water layer is slowly dripped with concentrated hydrochloric acid, Adjust the pH of the system to 5.0-5.5, filter, wash the filter cake with a large amount of water, and dry to obtain sulfadiazine with a yield of 96.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com