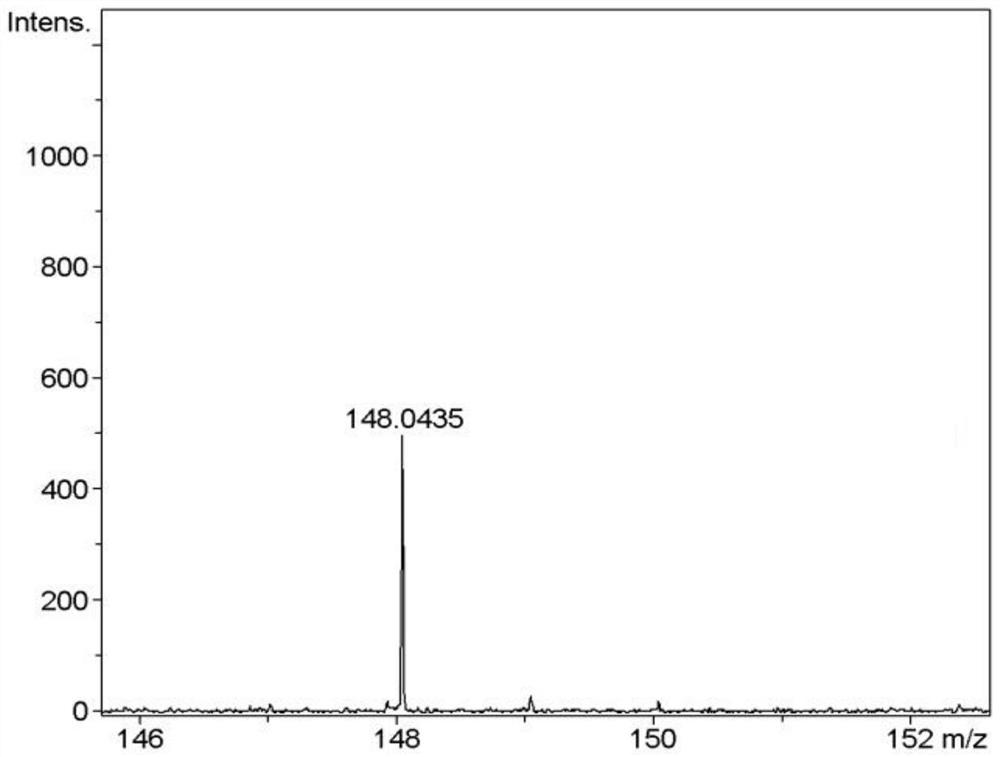
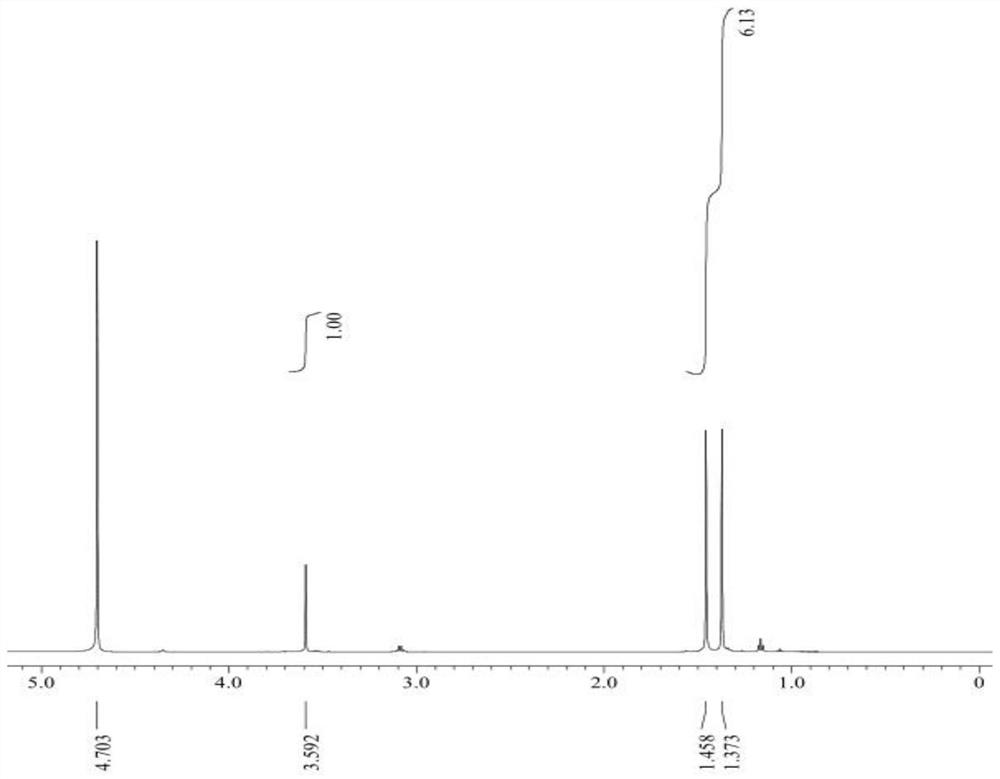
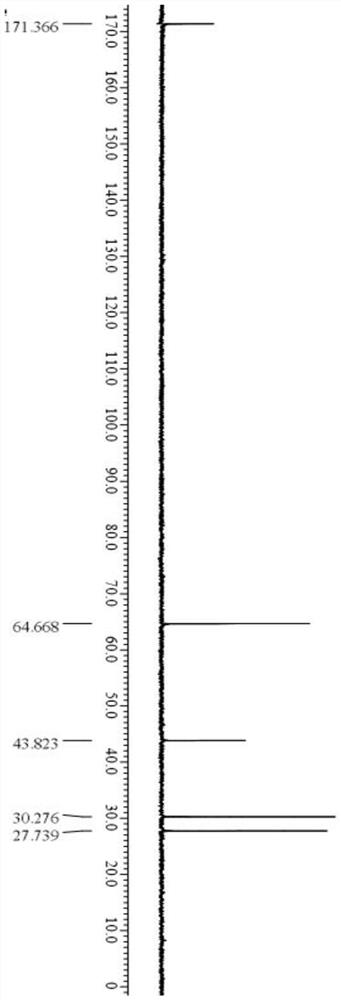
Preparation method of L-penicillamine
A technology of penicillamine and penicillamine hydrochloride, which is applied in the field of medicine and chemical industry, can solve the problems of high price of splitting reagent chloramphenicol, waste of raw materials and energy, manpower, and unsuitability for mass production, and achieve solvent recovery and application Convenient, easy to recycle and apply, low disassembly cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] The preparation method of L-penicillamine of the present invention, the corresponding chemical reaction process is as follows:
[0042]
Embodiment 1
[0044] A method for preparing L-penicillamine, steps S1 to S7 are carried out independently in respective reaction tanks, and intermediate products are separated from the reaction tanks after each step is completed, and then the intermediate products are used in the next step one step. Proceed as follows:
[0045] Synthesis of S1, isopropyl-D-penicillamine
[0046] Add 149.5 grams of D-penicillamine and 450 milliliters of acetone to a 1L three-necked bottle, stir and heat to 55°C, follow the reaction with a thin layer (n-butanol: glacial acetic acid: water volume ratio 4:1:1), 2 After one hour, the reaction was completed, and then concentrated under reduced pressure at room temperature to remove part of the acetone so that the volume of the reaction solution was reduced to one-third of the original volume, cooled to 5°C and stirred for 1 hour, filtered to obtain a white filter cake, dried in the air to obtain a white powder Isopropyl-D-penicillamine, weighing 172.8g, yield: ...
Embodiment 2
[0060] A method for preparing L-penicillamine, steps S1 to S4 are continuously completed in the same reaction tank, and the reaction tank does not need to be replaced midway. Proceed as follows:
[0061] S1. First, add 149.5g of D-penicillamine and 450ml of acetone into reaction tank A, heat to a temperature of 55°C, and track the reaction in a thin layer (n-butanol:glacial acetic acid:water volume ratio 4:1:1), After 2 hours, the reaction was completed, and then, the remaining acetone was removed by evaporation under reduced pressure at room temperature, and isopropyl-D-penicillamine was obtained in the reaction tank.
[0062] S2. Add 517.5ml of ethyl formate directly to the reaction tank of step S1, reflux reaction at a temperature of 53°C, and monitor the reaction with a thin layer (methanol:dichloromethane volume ratio 1:9). After 5 hours, the reaction is complete, and then reduce to normal temperature. The remaining ethyl formate was removed by pressure evaporation, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com