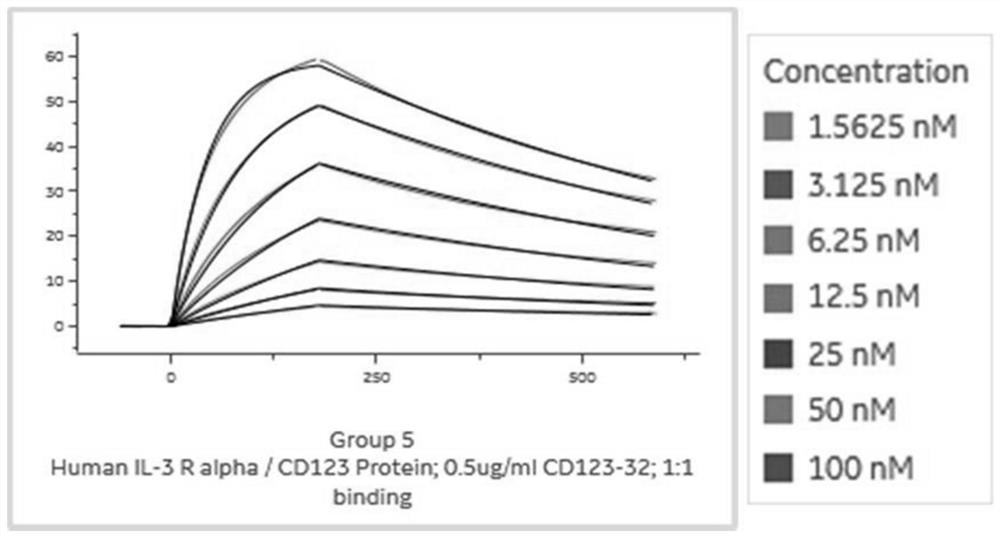
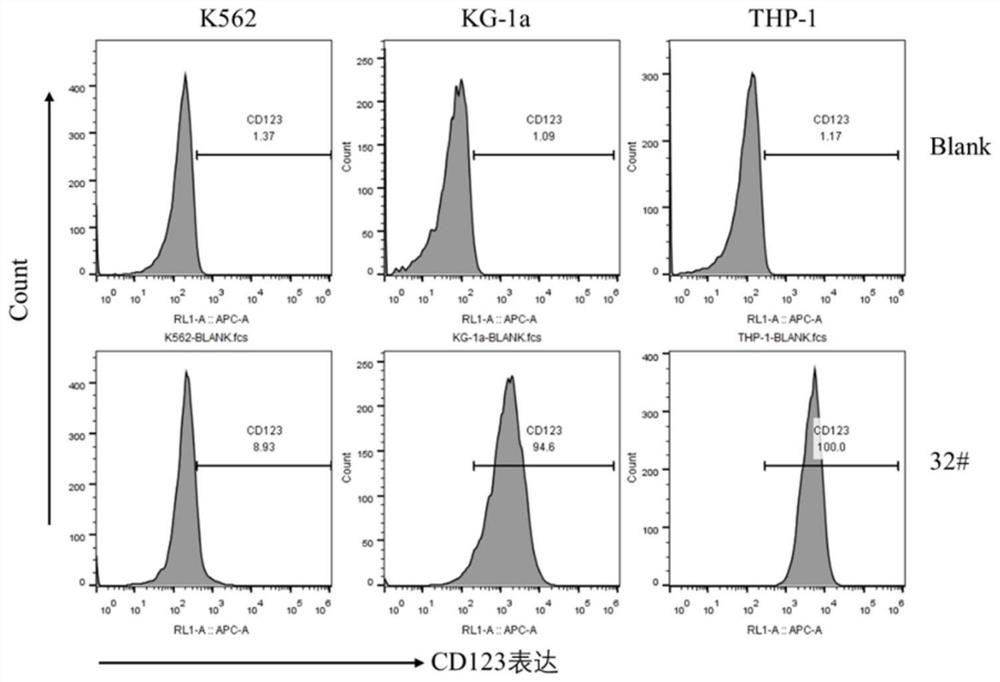
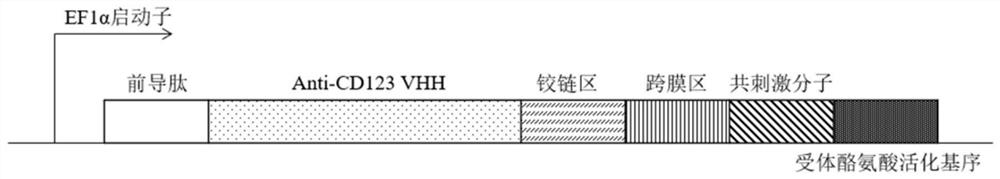
Anti-cd123 antibody and its application
A technology of antibodies and chimeric antigen receptors, applied in the direction of antibody medical components, antibody mimics/stents, carriers, etc., can solve problems such as failure to achieve ideals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1 Construction of phage antibody library, panning and preliminary screening by ELISA
[0065] (1) Construction of phage antibody library
[0066] In this example, the extracellular region of CD123 antigen was used to immunize Bactrian camels. After ELISA titer was verified, 200 mL of peripheral blood was extracted; lymphocytes were sorted from peripheral blood to obtain peripheral blood mononuclear lymphocyte precipitation, and RNA extraction was performed;
[0067] Using the extracted RNA as a template, III reverse transcriptase to synthesize the first-strand cDNA, then nested PCR was used to amplify the VHH gene; the amplified VHH gene was inserted into the pMECS phage display vector, TG1 competent cells were electrotransformed, and an appropriate amount of bacterial solution was taken for library identification. All cultures were spread evenly on LB / AMPGLU plates;
[0068] After the bacteria grow out, collect the bacterial moss, add 1 / 3 volume of 50% glyce...
Embodiment 2
[0081] Example 2 FACS screening of candidate clones
[0082] In this example, the cells were cultured according to the standard cell culture protocol:
[0083] Use trypsin to digest the cells to prepare a CD123-positive or CD123-negative cell suspension, centrifuge at 300g for 5 min to remove the culture medium, and resuspend the cells in Flow Buffer to a cell concentration of 2×10 6 pcs / mL;
[0084] Add 2 x 10 to each well of a V-bottom 96-well plate 5 Cells were centrifuged at 300 g for 5 min to remove the supernatant, and the crude VHH antibody extract was added to resuspend the cells, and incubated at 4°C for 1 h;
[0085] Centrifuge at 300 g for 5 min to remove the supernatant, resuspend the cells in Flow Buffer, add 100 μL of APC anti-his antibody (2 μg / mL) diluted in Flow Buffer, and incubate at 4°C for 1 h;
[0086] After washing the cells three times with Flow Buffer, resuspend the cells in 200 μL of Flow Buffer for flow cytometry.
Embodiment 3
[0087] Example 3 Expression, purification and affinity determination of VHH-mIgG2a Fc antibody
[0088] In order to further identify the screened antibodies, this example constructs a VHH-expressing vector C-4pCP.Stuffer-mCg2a-FC (with a mouse Fc tag), and the steps are as follows:
[0089] PCR amplification of the anti-CD123 heavy chain variable region encoding gene, wherein, the upstream primer of CD123-32 (SEQ ID NO: 5) is HD-F, and the downstream primer is HD-B8-R1, the sequence is shown in Table 2, PCR The reaction system is shown in Table 3. The reaction conditions were pre-denaturation at 95 °C for 1 min, denaturation at 95 °C for 10 s, annealing at 55 °C for 10 s, extension at 72 °C for 10 s, 30 cycles, extension at 72 °C for 5 min, and storage at 4 °C.
[0090] Table 2
[0091]
[0092] table 3
[0093]
[0094] The empty vector was digested at 37°C for 6 hours. The system was shown in Table 4. The digested vector was The PCR purification kit was purified an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com