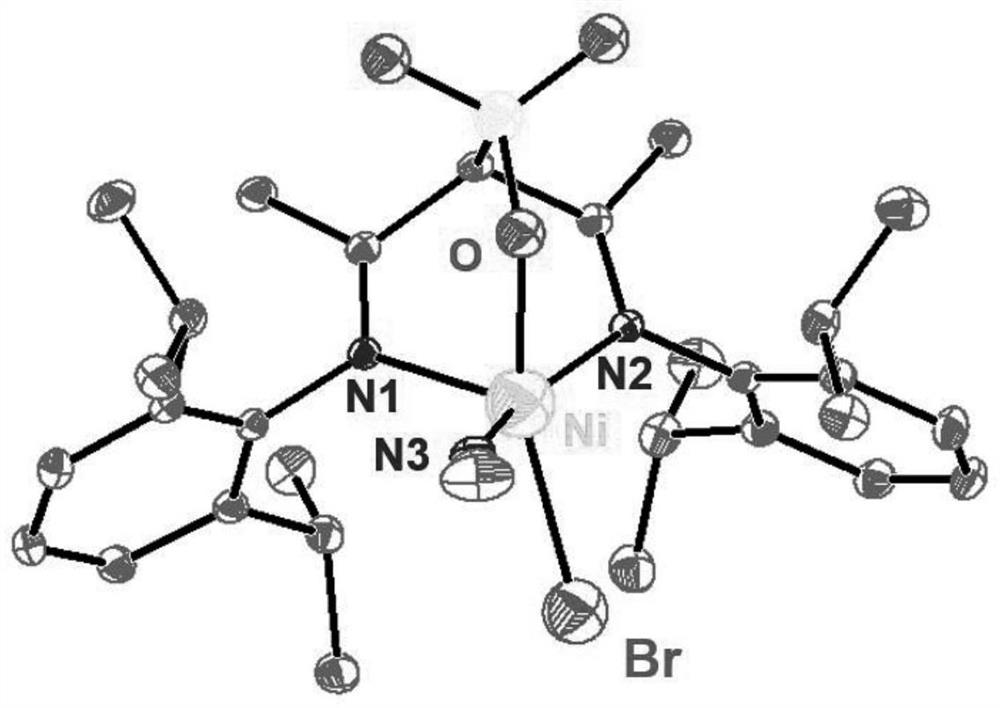
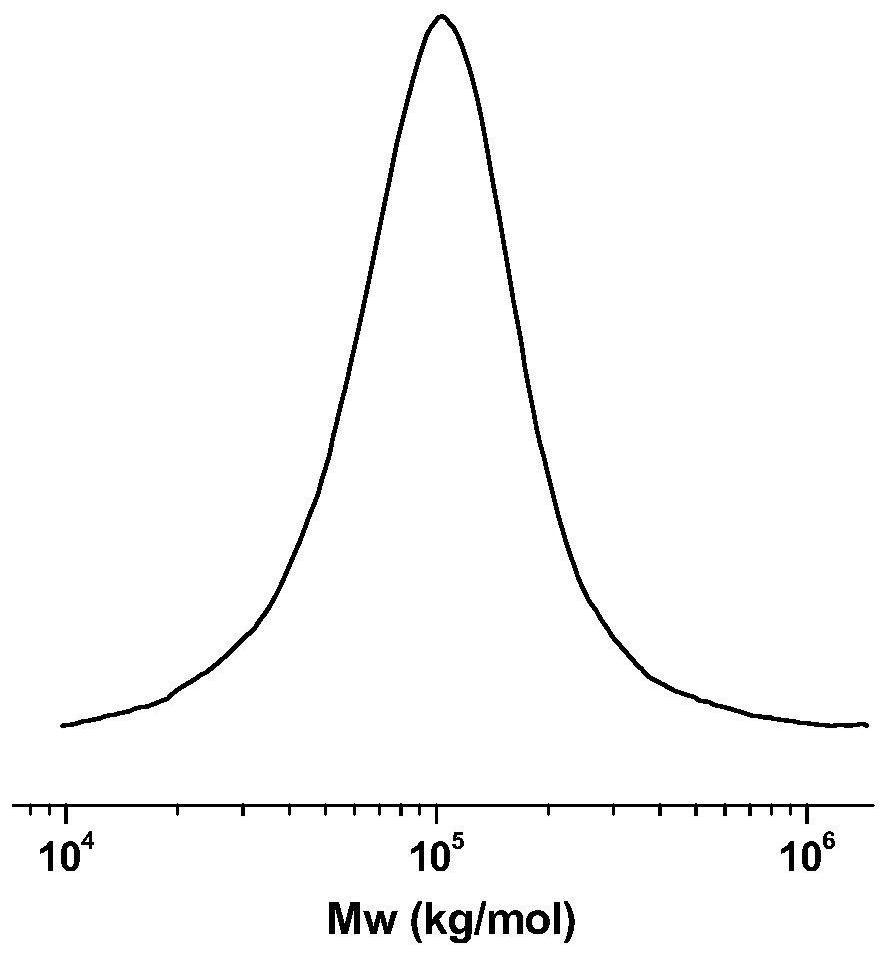
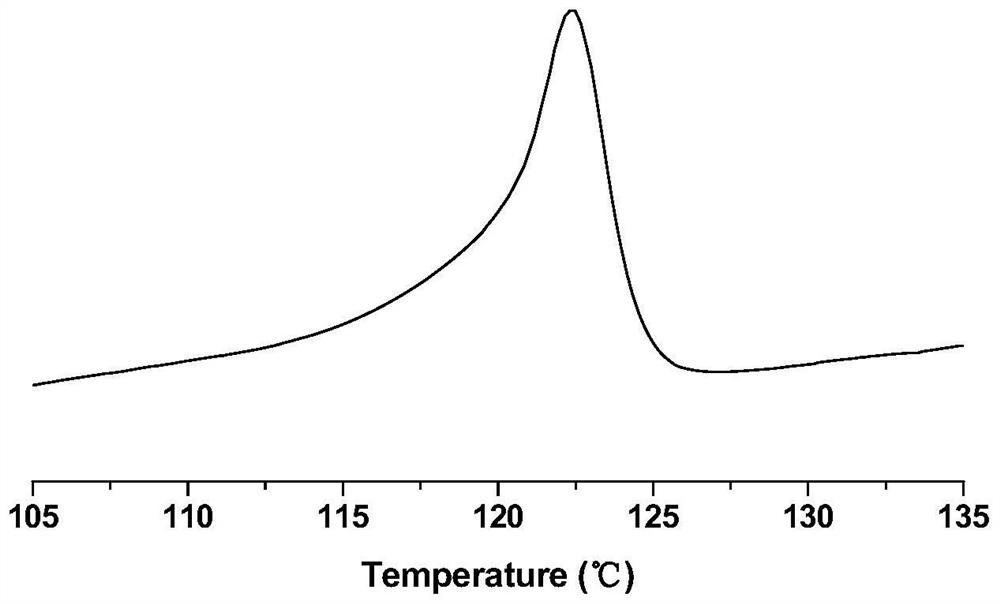
A kind of α-sulfonic acid-β-diimine nickel complex, its preparation method and its application in catalytic olefin polymerization
A nickel diimide, catalyzing olefin technology, applied in nickel organic compounds, organic chemical methods, chemical instruments and methods, etc., can solve the problems of poor thermal stability, low polymerization activity of phosphine sulfonate nickel palladium catalyst, low polymerization activity and the like , to achieve the effect of improved temperature resistance, good broad spectrum and high insertion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] This example provides a β-diimine A1 substituted with 2,6-diisopropylphenyl, and its synthesis method is as follows.
[0060] Acetylacetone (4.1g, 41mmol), 2,6-diisopropylaniline (15.1g, 85.2mmol), ethanol (500mL) and hydrochloric acid (12M, 6mL) were successively added into the round bottom flask. The system was heated to 100°C in an oil bath and reacted for 72 hours. Then the solution was cooled to room temperature, and the solvent was removed by rotary evaporation to obtain a brown solid. The resulting solid was dissolved in dichloromethane (300 mL), saturated NaHCO 3 The solution was washed and extracted twice, and the organic phase was collected. The organic phase was dried with anhydrous magnesium sulfate, filtered, and the filtrate was collected; then the filtrate was rotary evaporated to obtain a crude product, which was recrystallized with methanol to obtain a white solid. Yield 87.3%. The H NMR spectrum is as follows: 1 H NMR (CDCl 3, 400MHz) δ(ppm): 12...
Embodiment 2
[0062] This example provides a 2,6-dimethylphenyl substituted β-diimine A2, the synthesis method of which is as follows.
[0063] According to the synthesis method of Example 1, 2,6-dimethylaniline was replaced by 2,6-dimethylaniline to obtain a white solid. The yield was 82.7%. The H NMR spectrum is as follows: 1 H NMR (CDCl 3 ,400MHz)δ (ppm):12.17(s,1H,NH),7.05-6.92(m,6H,Ar-H),4.87(s,1H,H β ),2.16(s, 12H,CH 3 ),1.68(s,6H,α-CH 3 ).
Embodiment 3
[0065] This example provides an α-sulfonic acid-β-diimide lithium salt compound L1, the synthesis method of which is as follows.
[0066] Under nitrogen atmosphere, β-diimine A1 (3.86g, 9.24mmol) was dissolved in dry tetrahydrofuran, and n-BuLi (4.3mL, 10.75mmol) was slowly added dropwise at -78°C, and then stirred at -78°C for 1h , the resulting system was slowly warmed up to room temperature, and continued to stir for 30 min. -78°C, add SO 3 .NMe 3 (0.84g, 6.04 mmol), slowly rose to room temperature, and continued to stir for 24h. After the solution was filtered, the filtrate obtained was concentrated, frozen at -30°C to obtain a white precipitate, filtered to obtain a white solid, washed twice with n-hexane, and vacuum-dried to obtain a white solid with a yield of 82.3%. The H NMR spectrum is as follows: 1 H NMR(MeOD,400MHz)δ(ppm):7.13-7.02 (m,6H,Ar-H),5.04(s,1H,H β ),3.72(m,8H,THF),3.09(m,4H,CHMe 2 ),2.09(s,6H,CH 3 ),1.86(m,8H,THF),1.15(m,24H,CH(CH 3 ) 2 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com