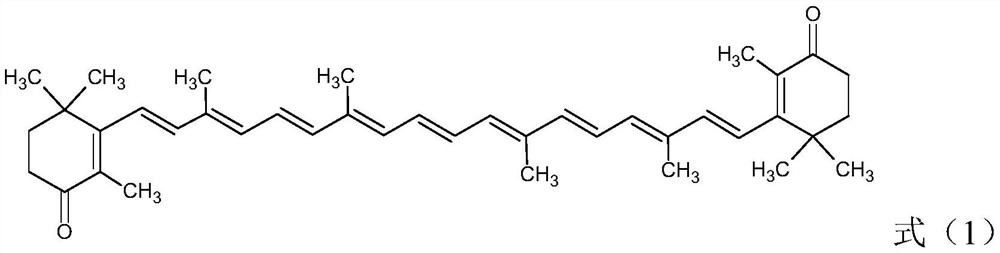
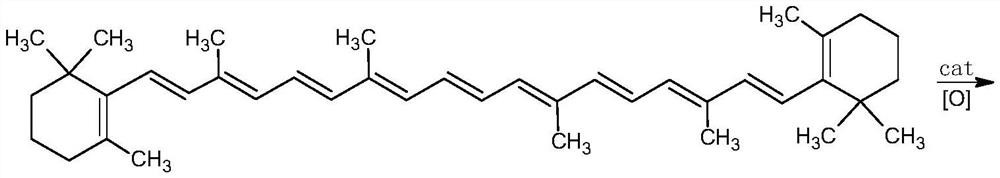
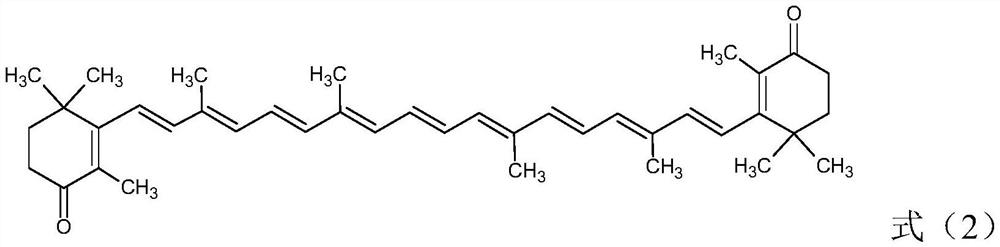
Cantharidin preparation method
A technology of cantharidin and carotene, which is applied in the directions of organic chemistry and chemical recovery, can solve the problems of low yield of cantharidin and large environmental pollution, and achieves the effects of good oxidation effect, high yield and reduced consumption.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Dissolve 0.30g (0.0018mol) of potassium iodide, 0.46g (0.0036mol) of manganese chloride, and 19.43g (0.1825mol) of sodium chlorate in 250mL of water, and adjust the pH value of the resulting aqueous solution to 4 with 5% dilute hydrochloric acid.
[0045] Add 20g of β-carotene (98.0%, 0.0365mol) and 500mL of dichloromethane into a 1000mL reactor, start stirring, add the above aqueous solution, pump nitrogen three times, cool down to 0-5°C, and keep warm for 3h. After the heat preservation is over, let it stand for stratification, wash the oil layer once with 150mL 2.5% w / v sodium thiosulfate solution, wash once with water, then concentrate to dryness, add 200mL ethanol, heat isomerization at 70°C for 8h, drop to room temperature, reduce Press filtration and dry to obtain 19.91 g of all-trans cantharidin with a content of 95.5% and a yield of 92.2%.
Embodiment 2
[0047] Dissolve 0.18g (0.0011mol) of potassium iodide, 0.28g (0.0022mol) of manganese chloride, and 11.66g (0.1095mol) of sodium chlorate in 250mL of water, and adjust the pH of the resulting aqueous solution to 4 with 5% dilute hydrochloric acid.
[0048] Add 20g of β-carotene (98.0%, 0.0365mol) and 500mL of dichloromethane into a 1000mL reactor, start stirring, add the above aqueous solution, pump nitrogen three times, cool down to 0-5°C, and keep warm for 3h. After the heat preservation is over, let it stand for stratification, wash the oil layer once with 150mL 2.5% w / v sodium thiosulfate solution, wash once with water, then concentrate to dryness, add 200mL ethanol, heat isomerization at 70°C for 8h, drop to room temperature, reduce After pressure filtration and drying, 19.71 g of all-trans cantharidin was obtained, the content was 90.5%, and the yield was 86.5%.
Embodiment 3
[0050] Dissolve 0.61g (0.0037mol) of potassium iodide, 0.92g (0.0073mol) of manganese chloride, and 19.43g (0.1825mol) of sodium chlorate in 250mL of water, and adjust the pH of the resulting aqueous solution to 4 with 5% dilute hydrochloric acid.
[0051] Add 20g of β-carotene (98.0%, 0.0365mol) and 500mL of dichloromethane into a 1000mL reactor, start stirring, add the above aqueous solution, pump nitrogen three times, cool down to 0-5°C, and keep warm for 3h. After the heat preservation is over, let it stand for stratification, wash the oil layer once with 150mL 2.5% w / v sodium thiosulfate solution, wash once with water, then concentrate to dryness, add 200mL ethanol, heat isomerization at 70°C for 8h, drop to room temperature, reduce After pressure filtration and drying, 20.11 g of all-trans cantharidin was obtained, the content was 93.2%, and the yield was 90.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com