Antibacterial hemostatic membrane as well as preparation method and application thereof
A technology of hemostatic film and thin film, applied in pharmaceutical formulation, pharmaceutical science, drug delivery, etc., can solve the problems of susceptibility to infection, loss of microsphere material, and difficulty of hemostatic microspheres to deal with large-area wounds, etc., and achieves short hemostasis time and rapid degradation. Speed, low-value effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
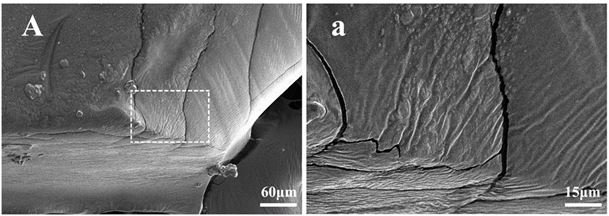
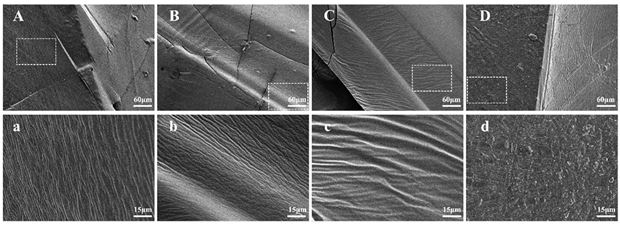
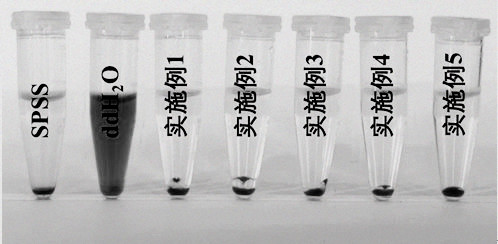
Image
Examples
Embodiment 1
[0037] 1. Preparation of matrix solution: Add 6 g of sodium alginate, 3 g of carboxymethyl chitosan, 0.15 g of collagen and 45 mL of glycerin to 555 ml of water for dissolving, and let stand at room temperature for 12 hours to remove bubbles inside the solution. Make 3 parts matrix solution.
[0038] 2. Cross-linking: Prepare 60 mL of calcium chloride solution with a mass concentration of 2%, and slowly pour the prepared matrix solution into an aluminum alloy container, spray the calcium chloride aqueous solution evenly with a watering can, carry out cross-linking reaction, and let stand After 15 min, transfer to a 37 °C incubator and let it stand for 48 h.
[0039] 3. Storage: Take a plastic template, cut it into several square films with a size of 10 cm×10 cm and a thickness of 1-2 mm, pack it in aluminum paper, weigh and seal it, and store it in a refrigerator at 4 °C.
Embodiment 2
[0041] 1. Preparation of matrix solution: Add 8 g sodium alginate, 4 g carboxymethyl chitosan, 0.2 g collagen, 0.12 g berberine hydrochloride and 60 mL glycerin to 740 ml water for dissolution, and let stand at room temperature for 12 h. Used to remove air bubbles inside the solution to make 4 parts of matrix solution.
[0042] 2. Cross-linking: Prepare 80 mL of calcium chloride solution with a mass concentration of 2%, and slowly pour the prepared matrix solution into an aluminum alloy container, spray the calcium chloride aqueous solution evenly with a watering can, carry out cross-linking reaction, and let stand After 15 min, transfer to a 37 °C incubator and let it stand for 48 h.
[0043] 3. Storage: Take a plastic template, cut it into several square films with a size of 10 cm×10 cm and a thickness of 1-2 mm, pack it in aluminum paper, weigh and seal it, and store it in a refrigerator at 4 °C.
Embodiment 3
[0045] 1. Preparation of matrix solution: 8 g sodium alginate, 4 g carboxymethyl chitosan, 0.2 g collagen, 0.36 g berberine hydrochloride and 60 mL glycerin were dissolved in 740 ml water respectively, and allowed to stand at room temperature for 12 h. Used to remove air bubbles inside the solution to make 4 parts of matrix solution.
[0046] 2. Cross-linking: Prepare 80 mL of calcium chloride solution with a mass concentration of 2%, and slowly pour the prepared matrix solution into an aluminum alloy container, spray the calcium chloride aqueous solution evenly with a watering can, carry out cross-linking reaction, and let stand After 15 min, transfer to a 37 °C incubator and let it stand for 48 h.
[0047] 3. Storage: Take a plastic template, cut it into several square films with a size of 10 cm×10 cm and a thickness of 1-2 mm, pack it in aluminum paper, weigh and seal it, and store it in a refrigerator at 4 °C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com