Method for evaluating Anti-infective drugs, vaccines, etc. using immortalized monocytic cells and induced cells
A mononuclear cell differentiation induction technology, applied in biochemical equipment and methods, botany equipment and methods, microorganisms, etc., can solve the problem of inability to obtain attenuated mutant strains, and achieve the effect of small error
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
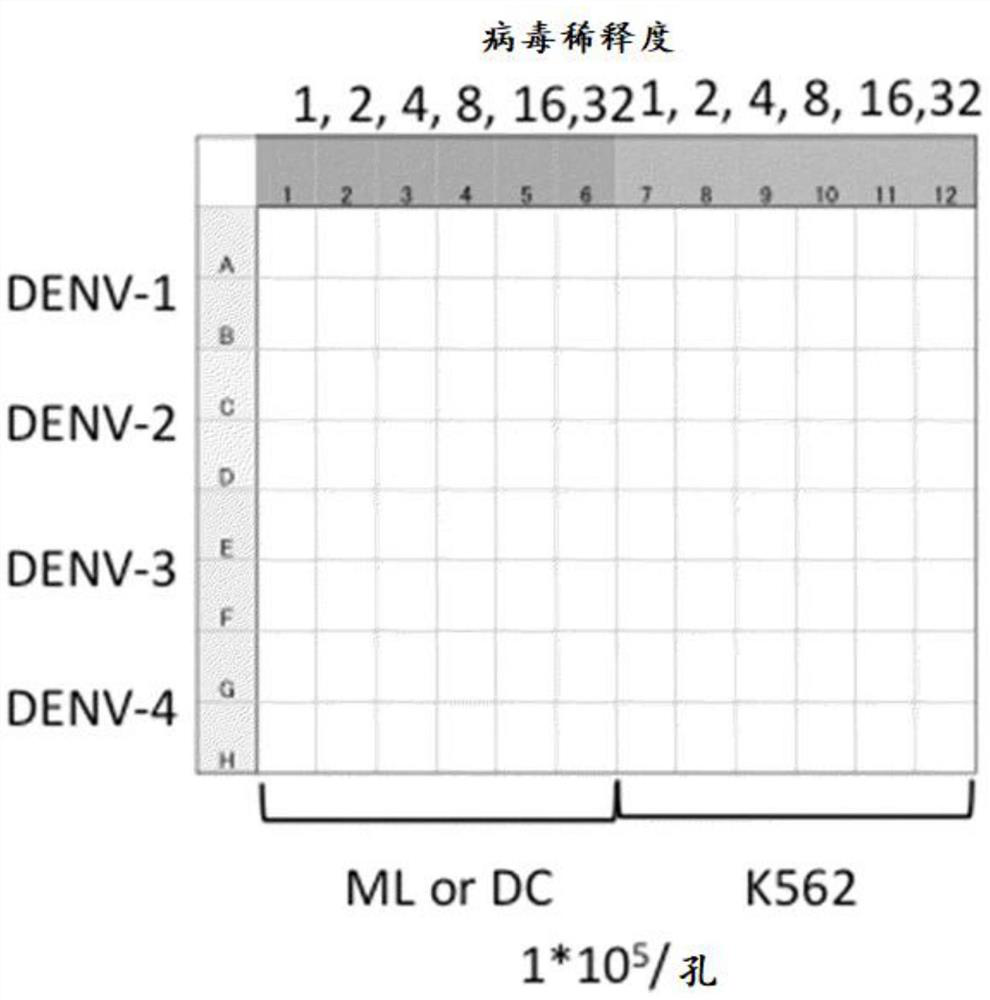
Examples
preparation example Construction
[0089]
[0090]In the present invention, a monocyte which becomes a raw material can be obtained from a method such as a fibroblast or the like from a plurality of cellular cell differentiated induced methods, from a plurality of stem cell differentiated induced methods. When a monocyte is extracted from a tip blood, it is known that a method of preparing a cell expressing CD14 molecule in human tipmed blood is known. For example, the use of normal saline, phosphate buffer, salt water or Hanks buffer, etc., using a physiological saline, phosphate buffer, and Hanks buffer. The diluted blood was carefully laminated on the FICOLL (Registered Trademark) (GE Healthcare) (GE Healthcare) (GE Healthcare), centrifugation at 15 to 30 ° C, 500 to 1000 × g. The yellow plasma with the transparent FICOLL intermediate white strip layer was recovered by a lymphocyte and monocytes. The recovered tip blood mononuclear cell component can also be used further after further cleaning. Cell cells can be obta...
Embodiment 1
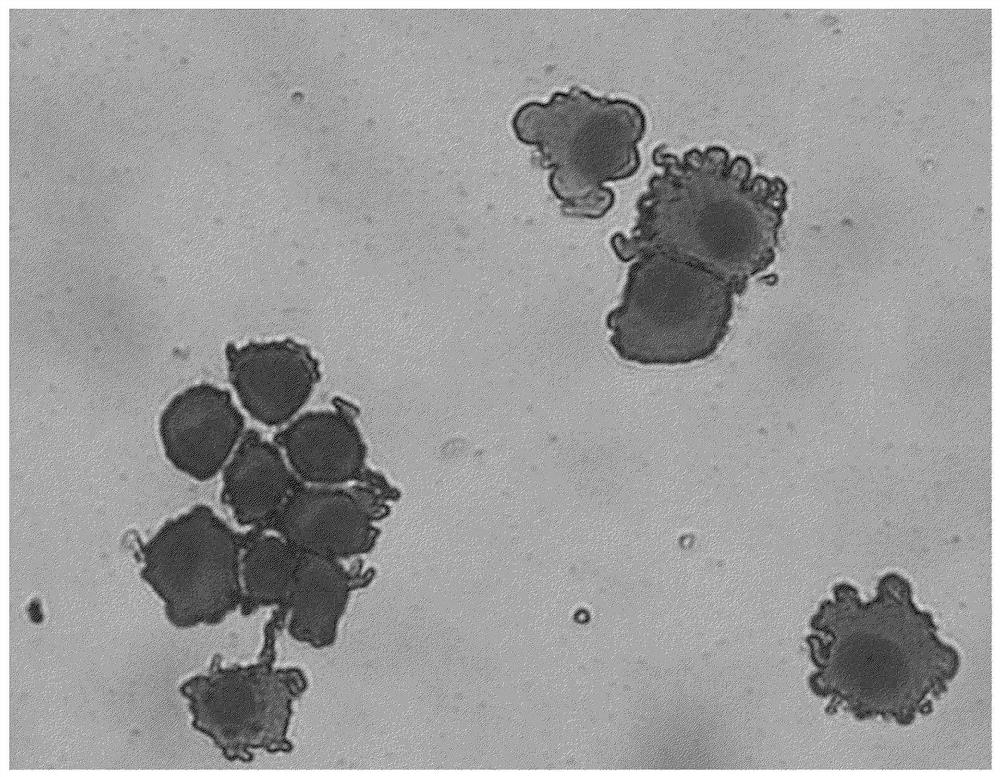
[0137](Example 1) Human Tip Source Permanent Monuclear Cell (CD14-ML) production and dendritic cell production (human tipped blood source immortal mononuclear cell production)
[0138]Human tipped sources of immortal mononuclear cells have been made (WO2012 / 043651 and Japanese special opening 2017-131136). In detail, the CD14 positive component is taken out from the end of the human tip and introduced as a gene that is reported as a mouse endogenous hematopoietic stem cell (Myc, Myb, HOB8, TLX1, E2A-PBX1, MLL, LJX2, RARA, HOXA9, NOT1, V -RAF / V-MYC, MyST3-NCoA2, EVI1, HOXB6, HOSB4, β-catenin, ID1), or genes in the forever-mononuclear cell report. The immortalized monocytes are cultured in α-MEM medium containing 20% FBS, 50 ng / ml m-CSF and 50 ng / ml GM-CSF, from starting to cultivate a proliferative cell, using commercially available Cell preservation fluid is stored in liquid nitrogen.
[0139](Confirmation of the obtained cells)
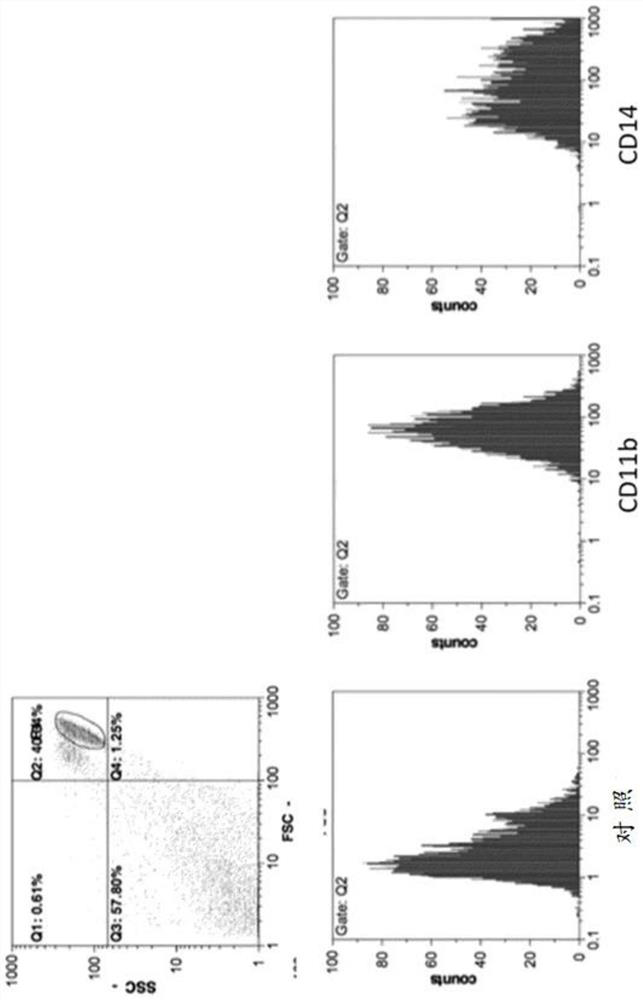
[0140]To confirm that the prepared cells are monocy...
Embodiment 2
[0145](Example 2) Made of immortal mononuclear cells (IPS-ML) from human induced multi-capable cells
[0146]Human-induced monocytes (IPS) sources of human immortal mononuclear cells have been made (WO2012 / 043651 and Japanese special opening 2017-131136). In detail, undifferentiated IPS cells were inoculated with substrate equipases such as laminin 511 (Laminin-511) as a base film, and the α-MEM medium (containing 20% FBS) was used. Differentiation induction culture. Then, a 1-day α-MEM medium (containing 20% FBS) continued to cultivate every 3 days. After 18 days of starting differentiation, Trypsin-Edta (ethylenediamine tetracetate)-collagenase (37 ° C, 60 minutes) cells, lysis, recovery, by pipetting (PIPETTING) Operation and production of cell suspensions. Next, the cells from a culture dish from one diameter of 10 cm were suspended in 10 ml of DMEM (containing 10% FBS) medium, and inoculated in two diameters without feeding cells and did not have gelatincoat. 10 cm of petriper...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com