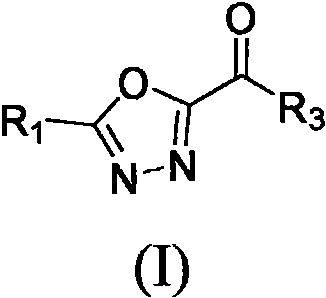
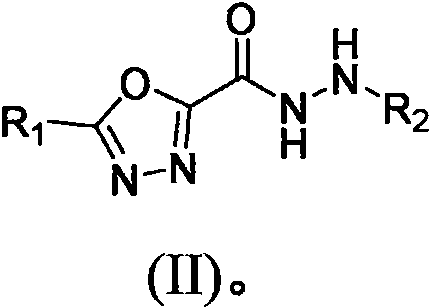
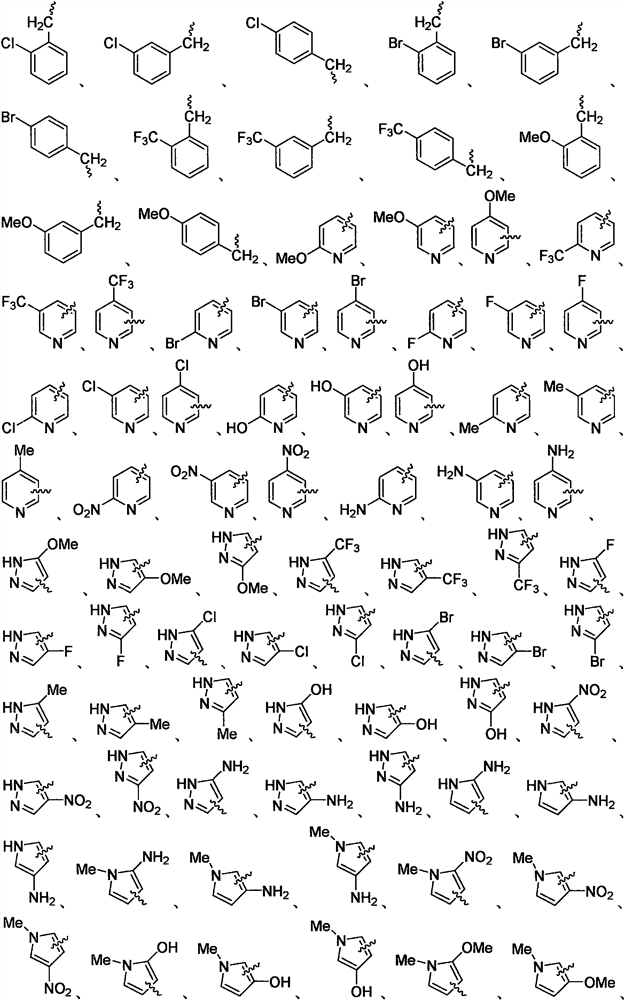
Heterocyclic substituted 1, 3, 4-oxadiazole hydrazide compound and preparation method and application thereof
A technology of oxadiazole hydrazide and compounds, applied in the field of medicinal chemistry, can solve the problems of farmers' economic losses, quality reduction, and crop yield decline
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1: Preparation of 3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid methyl ester
[0051] Dissolve 10.0g (56.8mmol) of 3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid in 100mL of methanol, then add concentrated sulfuric acid (8.0mL, 85.2mmol), at 75°C The reaction was stopped after reacting for 8 hours. After desolventization, 200 mL of ethyl acetate was added, washed with water 50 mL×3 times, dried over anhydrous sodium sulfate, and 10.5 g of a colorless oil was obtained after desolventization, with a yield of 97.2%.
[0052] For other ester intermediate compounds, corresponding raw materials or substituents are used, and are synthesized with reference to the steps of Example 1.
Embodiment 2
[0053] Example 2: Preparation of 3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carbohydrazide
[0054] Dissolve 10.5 g (55.2 mmol) of methyl 3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylate in 50 mL of anhydrous methanol, and add hydrazine hydrate (6.0 mL, 82.8 mmol) , 0 ° C reaction 10h to stop the reaction, add petroleum ether after precipitation, and then filter the solid, dry the solid to obtain a white solid 8.7g, yield 82.9%.
[0055] Other hydrazide intermediate compounds are synthesized by referring to the steps of Example 2 using corresponding raw materials or substituents.
Embodiment 3
[0056] Example 3: Preparation of 5-(3-(difluoromethyl)-1-methyl-1H-pyrazolyl)-1,3,4-oxadiazole-2-carboxylic acid methyl ester
[0057] Dissolve 5.0g (26.3mmol) of 3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carbohydrazide in 20mL of phosphorus oxychloride, then add methyl oxalyl chloride (3.6mL, 39.4 mmol), react at 85°C for 4h to stop the reaction, add 200mL of ethyl acetate after precipitation, wash 50mL×3 times, dry with anhydrous sodium sulfate, precipitation to obtain a brown solid, add ethanol to recrystallize to obtain a white solid 3.5g, yield 51.5 %. The melting point is 112.2-113.7°C.
[0058] For other ester intermediate compounds, corresponding raw materials or substituents are used, and are synthesized with reference to the steps of Example 3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com