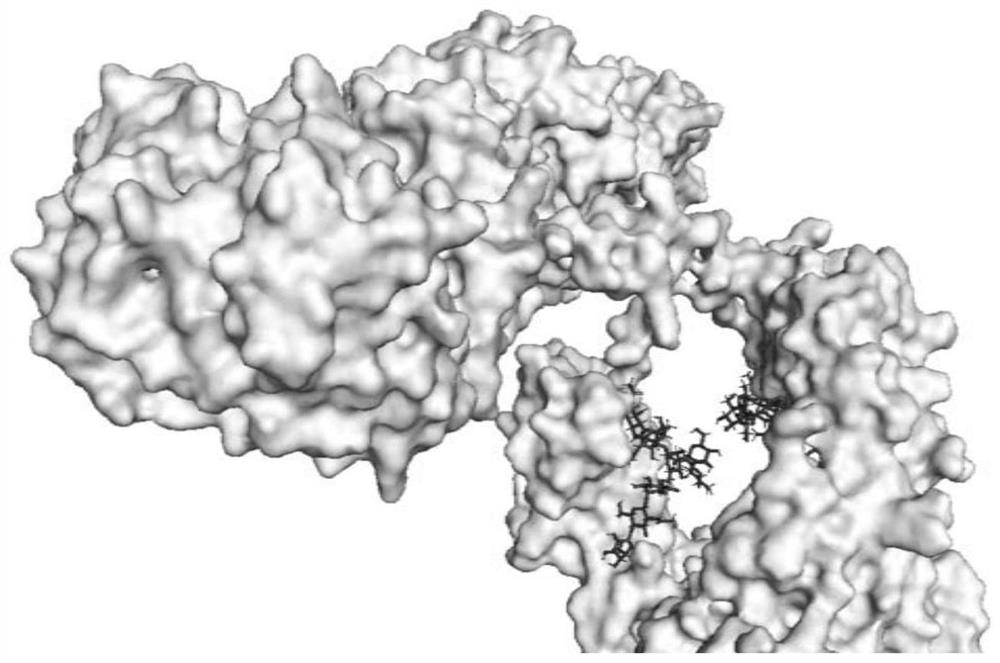
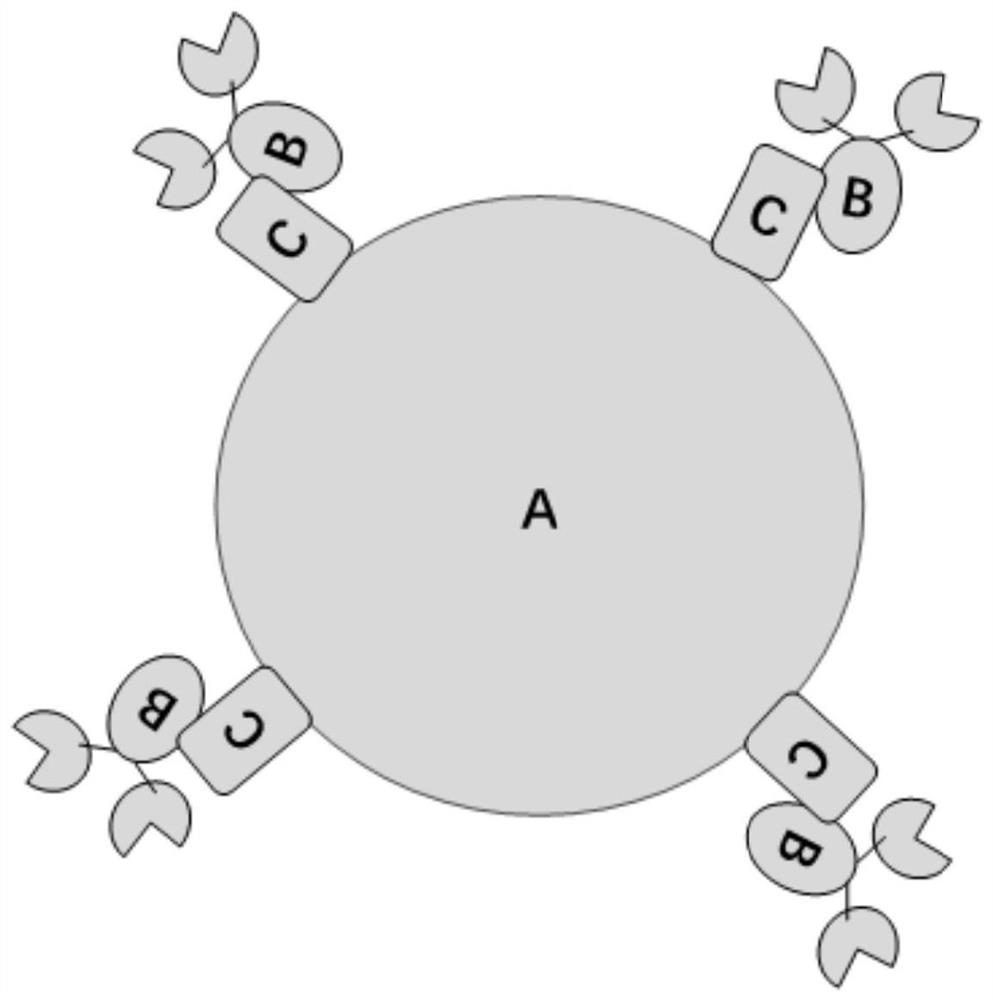
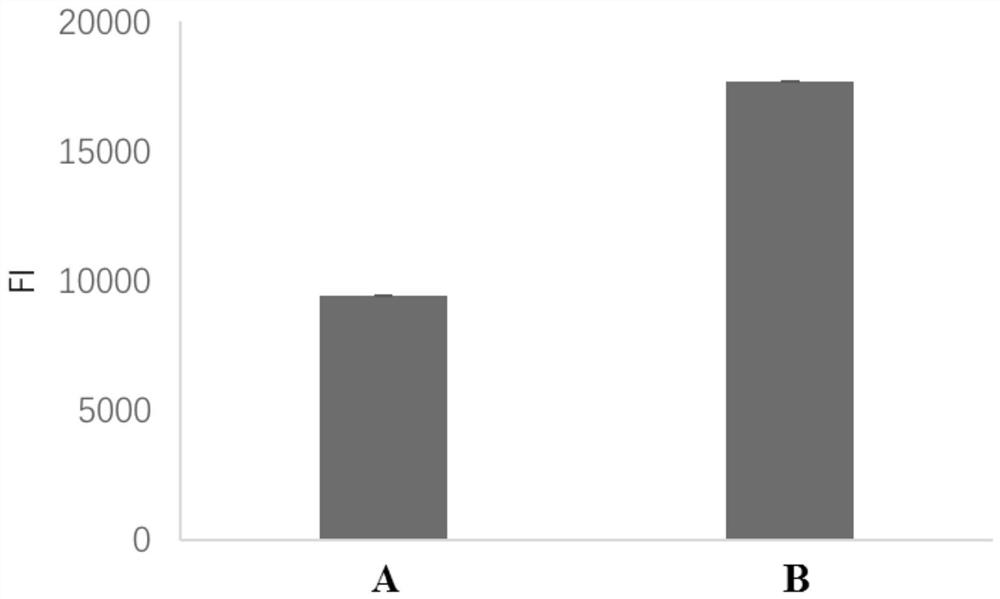
Method for realizing directional coupling by utilizing glycosyl of antibody and solid-phase carrier material
A solid-phase carrier and antibody technology, which is applied in the analysis of materials, material inspection products, and biological material analysis, can solve problems such as reducing the activity of antibody-binding antigens, blocking active sites, and affecting the stability and sensitivity of immunoassay methods. Effects of capture ability and extraction efficiency, increase in binding capacity, and increase in sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Directional coupling of antibody to aflatoxin B1 and time-resolved fluorescent microspheres (solid phase carrier material).
[0048] S1. Modification of time-resolved fluorescent microspheres:
[0049] 1) Take out 0.5mg of time-resolved fluorescent microspheres, centrifuge at 12000r for 15min at 4°C, and remove the protective solution;
[0050] 2) Add 0.2 mg of bovine serum albumin to the precipitate after centrifugation, and incubate at 25° C. for 2 hours. Centrifuge at 12000r for 15min at 4°C to remove excess bovine serum albumin. Wash twice with phosphate buffer (PB, 20mol / L, pH 7.2), centrifuge at 12000r for 15min at 4°C, and remove the liquid phase;
[0051] 3) Add 250 microliters of preservation solution (0.1% Proclin300) to the precipitate after centrifugation.
[0052] S2. Antibody activation:
[0053] 1) Prepare 2L dialysate 1mmol / L pH4.4 sodium acetate solution, add acetic acid to adjust the pH to 4.4, and pre-cool at 4°C;
[0054] 2) Prepare 0.1mol / L sod...
Embodiment 2
[0074] The antibody of C-peptide is coupled with immunomagnetic beads for the extraction of C-peptide in purified serum.
[0075] S0, magnetic bead pretreatment:
[0076] S0.1 Washing of Magnetic Beads
[0077] 1) Vortex to resuspend the carboxyl magnetic beads, pipette 100 μL magnetic beads into a 2 mL centrifuge tube, magnetically separate and discard the supernatant;
[0078] 2) Add 500 μL of coupling buffer (50 mmol / L MES, pH 6.0, 0.01% Triton X-100) to the tube, vortex for 20 s to wash the magnetic beads, place the centrifuge tube on the magnetic stand for 60 s, magnetically separate and discard clear; repeat the washing step three times;
[0079] Activation of S0.2 Magnetic Beads
[0080] 1) Prepare EDC (50mg / mL) and NHS (50mg / mL) with coupling buffer (ready-to-use), add 60 μL of coupling buffer, 20 μL of newly prepared EDC solution and 20 μL of newly prepared NHS solution, vortexed to mix.
[0081] 2) After incubating at room temperature for 15 minutes, place the c...
Embodiment 3
[0108] Directional coupling of zearalenone antibody to quantum dots for detection of zearalenone in edible oil.
[0109] S1. Quantum dot modification:
[0110] 1) Take out 0.5mg of quantum dots, centrifuge at 12000r for 15min at 4°C, and remove the protective solution;
[0111] 2) To activate, add 250 μL of MES buffer solution (50mmol / L, pH 5) containing 5mg EDC and 1.44mg NHS to the quantum dots, pipette up and down, shake at 25°C for 20min, centrifuge at 12000r for 15min at 4°C, and remove the liquid phase;
[0112] 3) Wash, add 250 μL of MES buffer (50 mmol / L, pH 5) to the precipitate after centrifugation, pipette up and down, sonicate for 3 minutes, centrifuge at 12000 r for 15 minutes at 4°C, and remove the liquid phase;
[0113] 4) Add 0.1 mg of bovine serum albumin to the precipitate after centrifugation, and incubate at 25° C. for 2 hours. Centrifuge at 12000r for 15min at 4°C to remove excess bovine serum albumin. Wash twice with phosphate buffer (PB, 20mmolL, pH 7...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com