Stable type NMN sustained-release micropellets, preparation method therefor and application of stable type NMN sustained-release micropellets
A sustained-release pellet and stable technology, which is applied in the field of stable NMN sustained-release pellets and its preparation, can solve the problems that NMN microcapsules do not have a sustained-release effect and the NMN activity has a large impact, and achieve excellent enteric-coated sustained-release Effects of performance, protective activity, ease of administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] The preparation method of the stable NMN sustained-release pellets provided by the invention comprises:
[0040] S1. Mix the core material and the stent wall material in a solvent to form a composite solution, and pass supercritical CO into the composite solution 2 fluid, at a temperature and pressure sufficient to make supercritical CO 2 The fluid is maintained under supercritical conditions for a period of time so that the supercritical CO 2 The fluid dissolves in the stent wall material so that the stent wall material expands, and then quickly releases the pressure so that the stent wall material solidifies around the core material to obtain NMN liposome nanospheres; the core material contains NMN, and the stent wall material contains Contains monoglycerides and phospholipids;
[0041] S2. Put the blank pellet core in the negative pressure bottom spray regular flow fluidized bed, and control the temperature in the negative pressure bottom spray regular flow fluidiz...
Embodiment 1
[0052] Formula composition
[0053] Formula composition Feeding amount / part NMN 9 glyceryl monolaurate 40 egg yolk lecithin 51 Microcrystalline Cellulose Blank Ball Core 75 glyceryl acetate 0.5 Ethyl cellulose 21 talcum powder 0.5
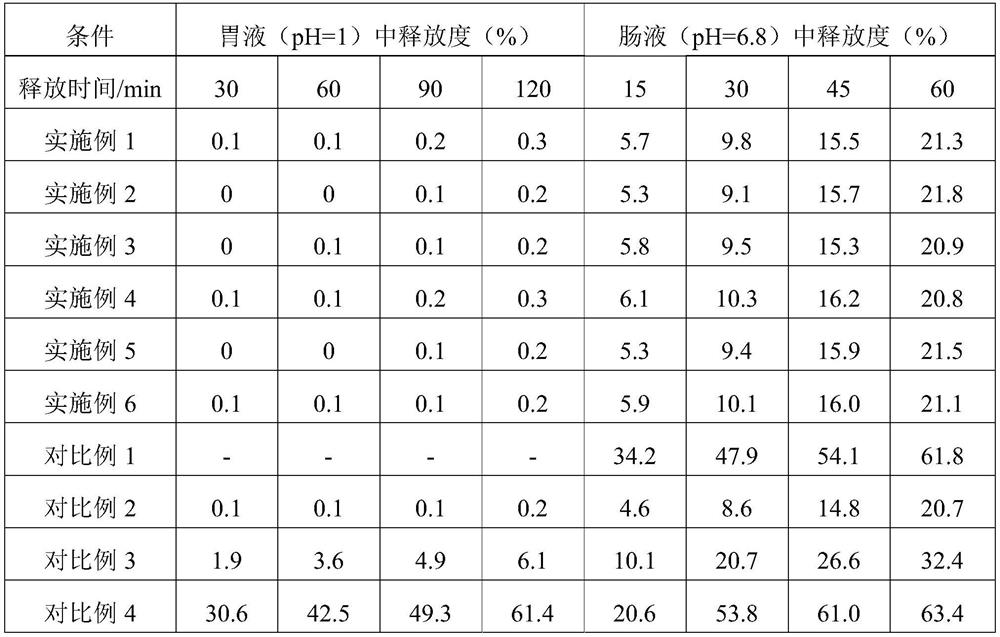
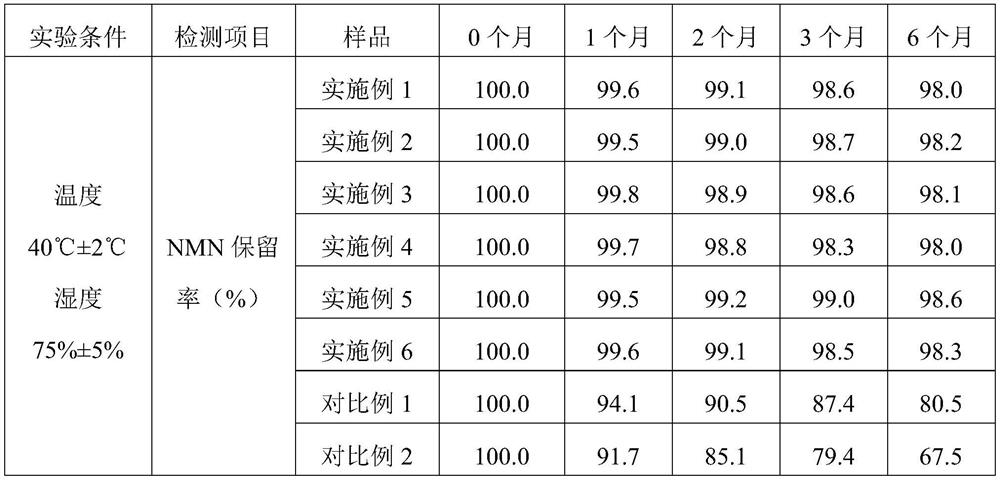
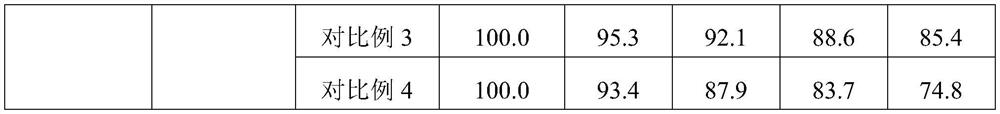
[0054] S1. Dissolve the NMN raw material with 1 part of water evenly, mix glycerol monolaurate and egg yolk lecithin evenly, then add 400 parts of ethanol solution, ultrasonically dissolve for 30 minutes, mix the above two solutions evenly and put them in an autoclave. Supercritical CO 2 Fluid, set the process parameters as follows: temperature 35°C, pressure 10MPa, time 10min, and then quickly release the pressure to obtain NMN liposome nanospheres.
[0055] S2. Place the microcrystalline cellulose blank pellet core in a negative pressure bottom spray regular flow fluidized bed, control the temperature in the negative pressure bottom spray regular flow fluidized bed at 30°C, adjust the air ...
Embodiment 2
[0057] Formula composition
[0058] Formula composition Feeding amount / part NMN 10 Glyceryl monostearate 30 Soy lecithin 60 Sucrose Blank Ball Core 70 Phthalates 3 Methacrylic acid-ethyl acrylate copolymer 15 talcum powder 1
[0059] S1. Dissolve the NMN raw material with 1 part of water evenly, mix glycerol monostearate and soybean lecithin evenly, add 150 parts of ethanol solution, ultrasonically dissolve for 30 minutes, mix the above two solutions evenly, put them in an autoclave, and into supercritical CO 2 Fluid, set the process parameters as follows: temperature 40°C, pressure 30MPa, time 15min, and then quickly release the pressure to obtain NMN liposome nanospheres.
[0060] S2, place the sucrose blank core in the negative pressure bottom spray regular flow fluidized bed, the temperature in the negative pressure bottom spray regular flow fluidized bed is controlled at 35°C, adjust the air flow of the fluidized b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com