Preparation method of diborane
A technology of diborane and boron trichloride, applied in the field of preparation of diborane, can solve the problems of long process and high cost, and achieve the effects of cost saving, raw material cost saving and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
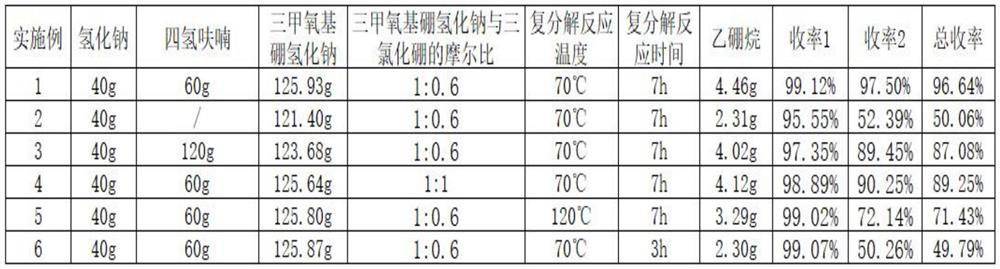
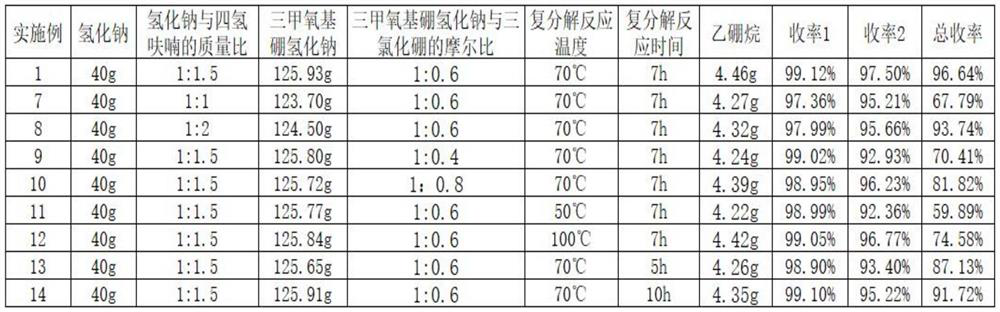
Embodiment 1
[0032] A preparation method for diborane, specifically comprising the following steps:
[0033] (1) Condensation reaction: Add 40g of sodium hydride to a 1000mL four-necked flask, pass nitrogen gas, use nitrogen to replace the air in the flask for 2 to 3 times, add 60g of tetrahydrofuran, stir, let stand for 1h, use nitrogen to dissolve tetrahydrofuran Press out the flask and repeat once. Take 200g of tetrahydrofuran, put it into the flask, slowly heat up the oil bath to 68°C, slowly add 109.12g of trimethyl borate dropwise, the dropwise addition is completed in 1.5h, keep it under full reflux for 5h, the sodium hydride solution turns from silvery white to off-white, and 125.93g is obtained Sodium trimethoxyborohydride, the yield is 99.12% based on sodium hydride.
[0034] (2) Metathesis reaction: control the temperature of the mixed solution of sodium trimethoxyborohydride tetrahydrofuran to 70°C, slowly add 384.48 g of 20% boron trichloride tetrahydrofuran solution dropwise...
Embodiment 2
[0038] A preparation method for diborane, specifically comprising the following steps:
[0039] (1) Condensation reaction: Add 40g of sodium hydride into a 1000mL four-neck flask, replace with nitrogen for 2 to 3 times, then slowly raise the temperature to 68°C, slowly add 109.12g of trimethyl borate dropwise, complete the dropwise addition in 1.5h, and keep warm under full reflux After 5 hours, the sodium hydride solution changed from silvery white to off-white to obtain 121.40 g of sodium trimethoxyborohydride, with a yield of 95.55% based on sodium hydride.
[0040] (2) Metathesis reaction: control the temperature of the mixed solution of sodium trimethoxyborohydride tetrahydrofuran to 70°C, slowly add 335.93 g of 20% boron trichloride tetrahydrofuran solution dropwise, and complete the dropwise addition in 1.5 hours, and keep it under total reflux for 7 hours to obtain diborane Crude. Use a three-stage ultra-low temperature vent condenser to cool, and the cooling temperat...
Embodiment 3
[0044] A preparation method for diborane, specifically comprising the following steps:
[0045] (1) Condensation reaction: Add 40g of sodium hydride to a 1000mL four-neck flask, replace with nitrogen 2 to 3 times, add 120g of tetrahydrofuran, stir, let stand for 1h, use nitrogen to press tetrahydrofuran out of the flask, and repeat once. Take 200g of tetrahydrofuran, put it into the flask, slowly heat up the oil bath to 68°C, slowly add 109.12g of trimethyl borate dropwise, the dropwise addition is completed in 1.5h, keep it under full reflux for 5h, the sodium hydride solution turns from silvery white to off-white, and 123.68g is obtained Sodium trimethoxyborohydride, the yield is 97.35% based on sodium hydride.
[0046] (2) Metathesis reaction: Control the temperature of the mixed solution of sodium trimethoxyborohydride tetrahydrofuran to 70°C, slowly add 342.25 g of 20% boron trichloride tetrahydrofuran solution dropwise, and complete the dropwise addition in 1.5 hours, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com