Preparation method of medicine for treating chronic hyperuricemia
A technology for hyperuricemia and medicine, applied in the medical field, can solve the problems of unsuitability for industrial production, high operational risk, and high chloride content, and achieve the effects of solving the problem of excessive chloride, high energy consumption, and high yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The preparation of embodiment 1 topicastat
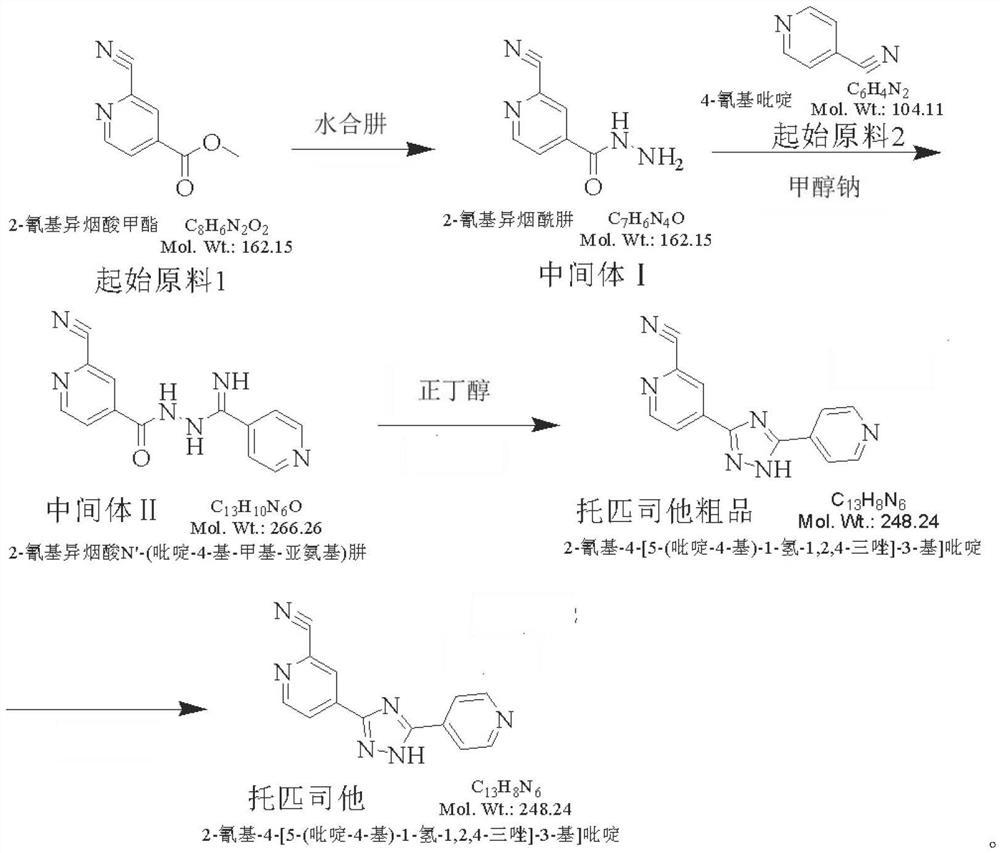
[0026] Add 20.0kg of methyl 2-cyanoisonicotinate and 200kg of absolute ethanol into a 500L reaction kettle, control the temperature to 25-30°C, add hydrazine hydrate ethanol solution dropwise under stirring, and continue to stir for 2 hours at a temperature of 30-35°C. Concentrate the reaction solution under reduced pressure, evaporate to dryness at a temperature of 45-55°C; add 60kg of dichloromethane, stir for 1 hour, filter with suction at room temperature, rinse the filter cake with 15kg of ethyl acetate, drain it, and place the filter cake in a blast oven for After drying at 65°C for 12 hours, 14.3 kg of intermediate I was obtained.
[0027] Add 8.233kg of 4-cyanopyridine and 65kg of methanol into a 200L reaction kettle, add 641g of sodium methoxide under stirring, control the temperature at 25-30°C for 2 hours, add 14.25kg of intermediate I in batches, control the temperature at 25-35°C, After stirring and reacting for...
Embodiment 2
[0031] The preparation of embodiment 2 topicastat
[0032] Add 20.0kg of methyl 2-cyanoisonicotinate and 200kg of absolute ethanol into a 500L reaction kettle, control the temperature to 25-30°C, add hydrazine hydrate ethanol solution dropwise under stirring, and continue to stir for 2 hours at a temperature of 30-35°C. Concentrate the reaction solution under reduced pressure, evaporate to dryness at a temperature of 45-55°C; add 60kg of dichloromethane, stir for 1 hour, filter with suction at room temperature, rinse the filter cake with 15kg of ethyl acetate, drain it, and place the filter cake in a blast oven for After drying at 65°C for 12 hours, 14.3 kg of intermediate I was obtained.
[0033] Add 8.233kg of 4-cyanopyridine and 65kg of methanol into a 200L reaction kettle, add 641g of sodium methoxide under stirring, control the temperature at 25-30°C for 2 hours, add 14.25kg of intermediate I in batches, control the temperature at 25-35°C, After stirring and reacting for...
Embodiment 3
[0037] The preparation of embodiment 3 topicastat
[0038] Add 20.0kg of methyl 2-cyanoisonicotinate and 200kg of absolute ethanol into a 500L reaction kettle, control the temperature to 25-30°C, add hydrazine hydrate ethanol solution dropwise under stirring, and continue to stir for 2 hours at a temperature of 30-35°C. Concentrate the reaction solution under reduced pressure, evaporate to dryness at a temperature of 45-55°C; add 60kg of dichloromethane, stir for 1 hour, filter with suction at room temperature, rinse the filter cake with 15kg of ethyl acetate, drain it, and place the filter cake in a blast oven for After drying at 65°C for 12 hours, 14.3 kg of intermediate I was obtained.
[0039] Add 8.233kg of 4-cyanopyridine and 65kg of methanol into a 200L reaction kettle, add 641g of sodium methoxide under stirring, control the temperature at 25-30°C for 2 hours, add 14.25kg of intermediate I in batches, control the temperature at 25-35°C, After stirring and reacting for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com