Metal organic framework material for selectively and quickly removing Sr2+ in strong alkaline solution and preparation and application thereof
A metal-organic framework, CH2PO3 technology, applied in other chemical processes, chemical instruments and methods, adsorption water/sewage treatment, etc., can solve problems such as poor selectivity, slow removal kinetics, and insufficient performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: the synthesis of SZ-7
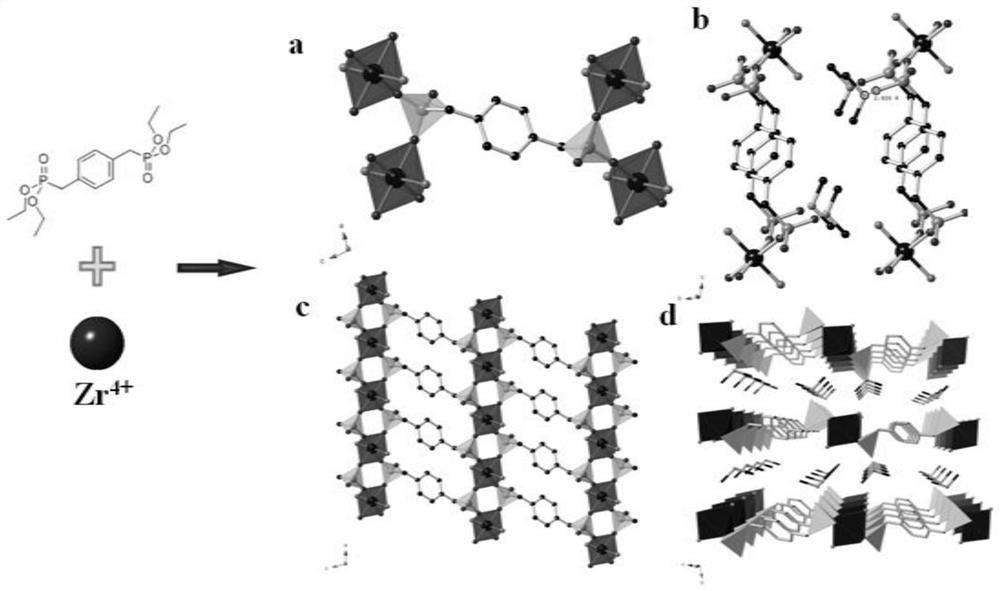
[0036] ZrOCl 2 ·8H 2 O (48.3mg, 0.15mmol), tetraethyl p-xylene diphosphate (C 16 h 28 o 6 P 2 ) (94.5mg, 0.25mmol), commercially available concentrated nitric acid (100μL), commercially available concentrated hydrofluoric acid (50μL) and a mixed solvent of 2.5ml water and DMA (V DMA :V 水 =4:1) sealed in a 10mL tetrafluoroethylene reactor, then heated in an oven at 210°C for 3 days, then dropped to room temperature at a cooling rate of 23°C / h, after the reaction was completed, the precipitate was washed with water and ethanol, After drying, a colorless single-phase SZ-7 single crystal was obtained.
[0037] Schematic diagram of the synthesis and structure of SZ-7 figure 2 As shown, the whole material is composed of negative zirconium phosphonate layers and interlayer cations to form a three-dimensional structure.
[0038] SZ-7 is a single crystal material, and its various parameters are shown in Table 1:
[0039] Table 1. ...
Embodiment 2
[0042] Embodiment 2: the stability test of SZ-7
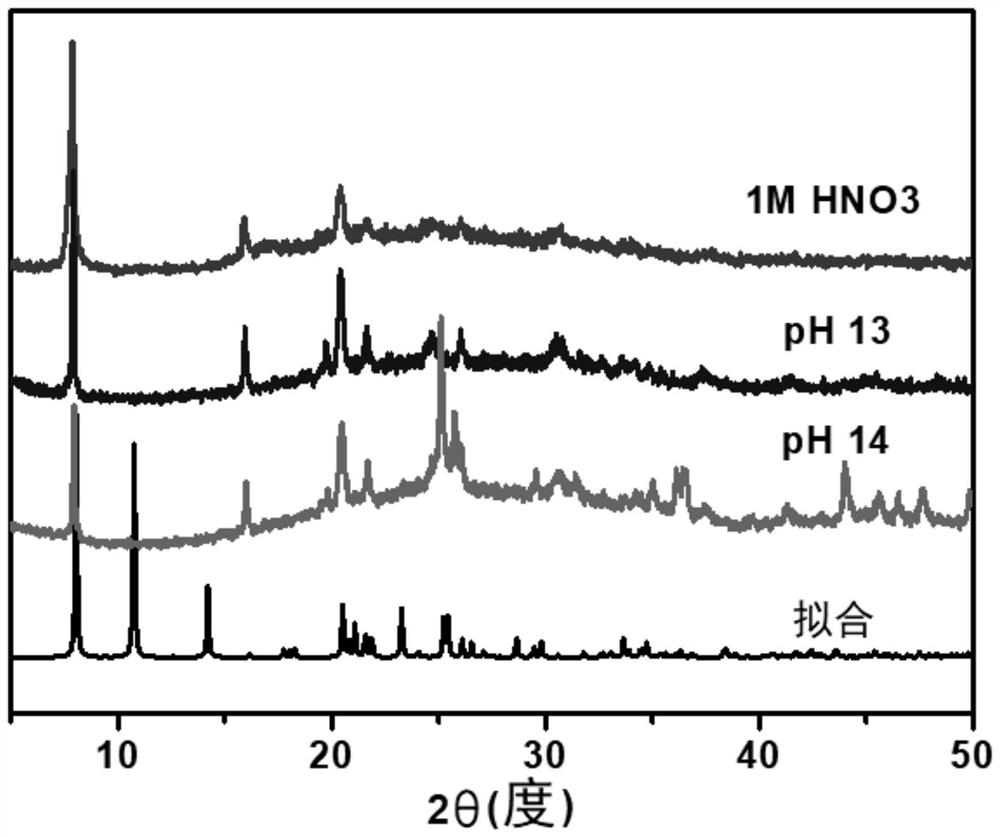
[0043] Before studying the actual removal ability of the material, the water stability of the material was studied first. SZ-7 was treated with an aqueous solution with a pH of 0-14. Specifically, the pH of the aqueous solution was adjusted to 0-14 by nitric acid and sodium hydroxide solution. Add 10 mg of SZ-7 to 10 mL of the above aqueous solution and shake for 1 day, then collect the solid and dry it for PXRD (powder X-ray diffraction) test, as shown in figure 2 As shown, the curves from top to bottom in the figure are 1M concentrated nitric acid (pH=0), pH13, pH14, and crystal PXRD curves under fitting conditions. The results show that SZ-7 can exist stably at pH 0-14, In the extreme case Sr 2+ The removal laid the foundation.
Embodiment 3
[0044] Embodiment 3: SZ-7 removes Sr 2+ test results
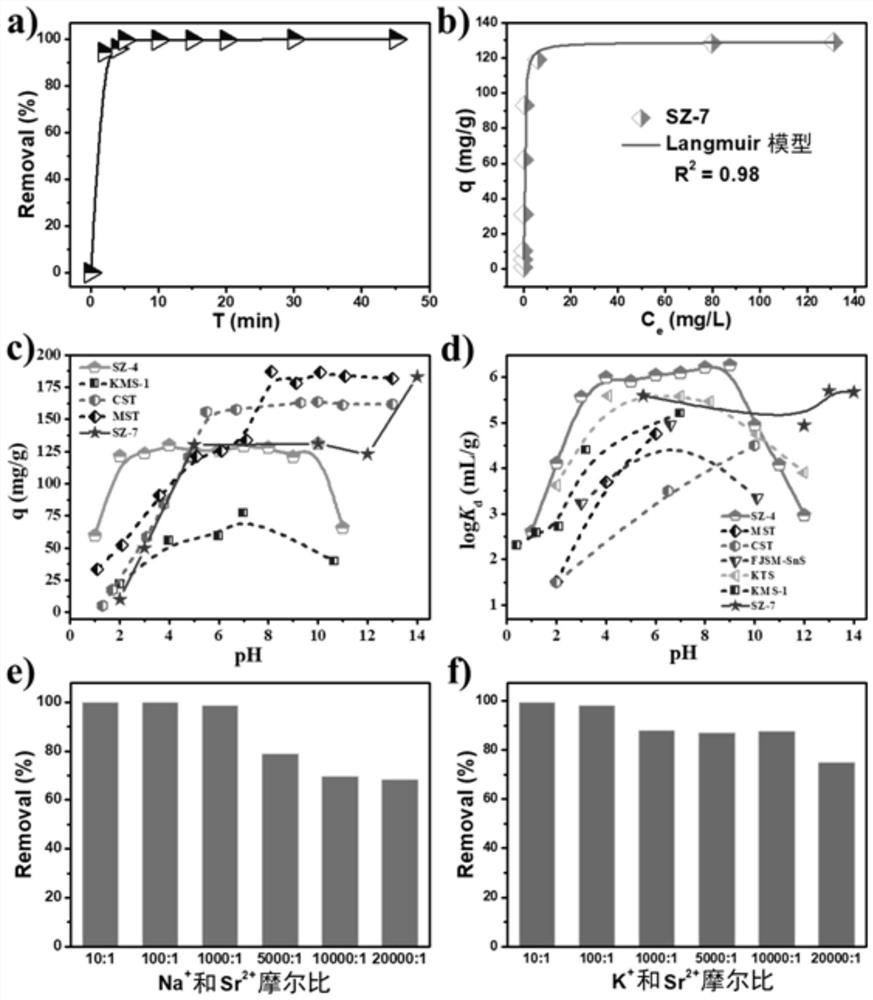
[0045] At room temperature, put SZ-7 into Sr-containing 2+ In aqueous solution, in solution, Sr 2+ The concentration is 10ppm, and the dosage of SZ-7 is 1g / L. Test SZ-7 on Sr at different time points 2+ The removal rate, the result is as follows image 3 a) as shown. SZ-7 vs. Sr 2+ The removal kinetics results ( image 3 a)) shows that SZ-7 can remove 94% of Sr in aqueous solution within 2 minutes 2+ , which in Sr 2+ The removal of materials is the fastest, surpassing most materials, including commercial and traditional Sr 2+ Remove material. The inner mechanism of ultrafast kinetics is related to the channel matching principle, the interlayer distance and Sr of SZ-7 2+ hydrated ionic radius Highly matched so that SZ-7 can remove most of Sr in water in extreme time 2+ . The fast kinetics can also reduce the contact time between SZ-7 and the extreme water environment, and reduce the damage to materials caused...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com