Nitrogen-containing organic compound and electronic component and electronic device using same
A technology of organic compounds and carbon number, applied in the field of organic electroluminescence, to avoid the reduction of triplet energy level, high electron mobility, improve service life and luminous efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0148] According to one embodiment, the electronic component is an organic electroluminescent device. The organic electroluminescent device may be, for example, a red organic electroluminescent device.
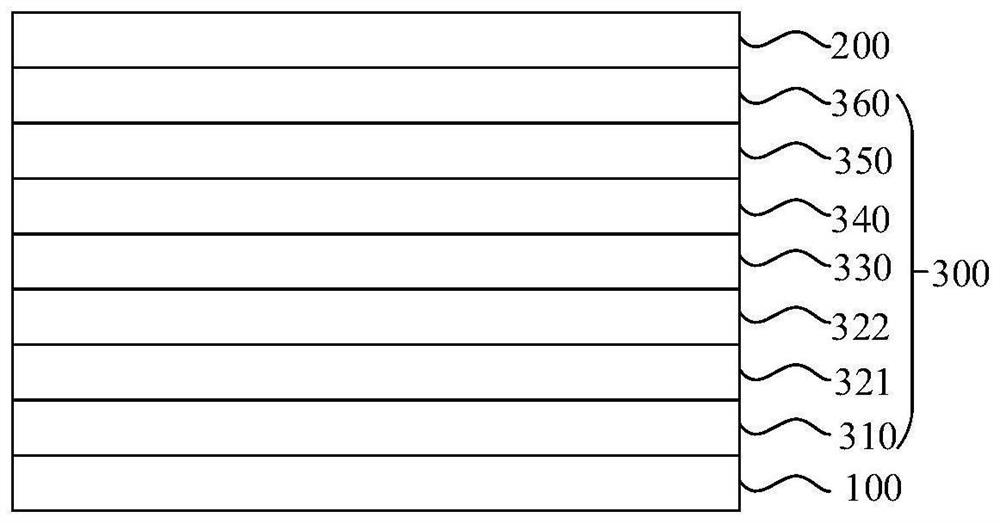
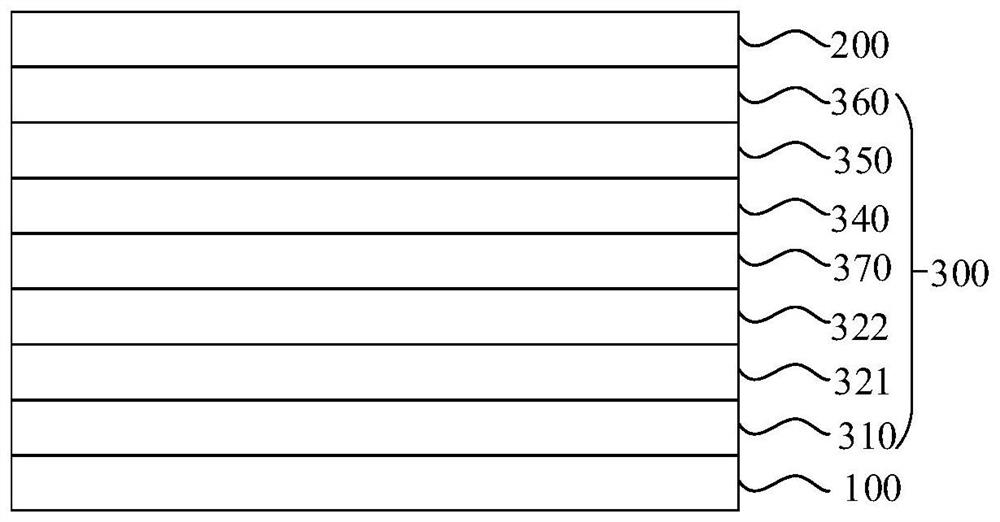
[0149] In a specific embodiment of the present application, the functional layer includes an electron transport layer, and the electron transport layer includes the nitrogen-containing organic compound. The electron transport layer may be composed of the nitrogen-containing organic compound provided by the present disclosure, or may be composed of the nitrogen-containing organic compound provided by the present application and other materials. The electron transport layer may be one layer or two or more layers.
[0150] In a specific embodiment, the organic electroluminescence device may include an anode 100, a hole transport layer 321, an electron blocking layer 322, an organic electroluminescence layer 330 as an energy conversion layer, an electron transport layer 350 and ...
preparation example 1
[0179] Synthesis of Preparation Example 1 Intermediate Sub 1-I-AX
[0180] in N 2 Under protection, magnesium flakes (6.7 g, 278.9 mmol) and 30 mL of tetrahydrofuran solution were added to the three-necked flask, the temperature of the system was raised to 60° C., and iodine (0.9 g, 3.5 mmol) was added to the system. The compound 1-bromoadamantane (50.0 g, 232.4 mmol) was completely dissolved in 480 mL of solution, and was slowly added dropwise to the system within 30 min, and the temperature was controlled at 60° C. during the dropwise addition. After the dropwise addition was completed, the reaction was stirred at a temperature of 60 °C for 2 h. After cooling at room temperature, cyanuric chloride (42.8 g, 232.4 mmol) dissolved in 40 mL of THF was added dropwise to the mixed solution, and the reaction was terminated after stirring for 3 h. After the reaction, toluene (200 mL) and water (100 mL) were added to extract the reaction solution, the organic phases were combined, ...
preparation example 2
[0183] Preparation example 2 Synthesis of intermediate sub 1-AX
[0184] Sub 1-I-A1 (20.0 g, 70.4 mmol), phenylboronic acid (8.6 g, 70.4 mmol), tetrakistriphenylphosphine palladium (4.2 g, 3.5 mmol), potassium carbonate (19.4 g, 140.7 mmol), tetrakis Butylammonium bromide (0.2 g, 0.7 mmol), toluene (160 mL), ethanol (80 mL) and deionized water (40 mL) were added to a three-necked flask, heated to 78°C under nitrogen protection, heated under reflux and stirred for 15 h. After the reaction, the solution was cooled to room temperature, toluene and water were added to extract the reaction solution, the organic phases were combined, the organic layer was dried over anhydrous magnesium sulfate, filtered, and concentrated; the crude product was purified by silica gel column chromatography to obtain a solid intermediate sub 1-A1 (15.4 g, 67%). The reaction process is shown in formula (3).
[0185]
[0186] The intermediate sub 1-AX was prepared in the same manner as in Preparatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com