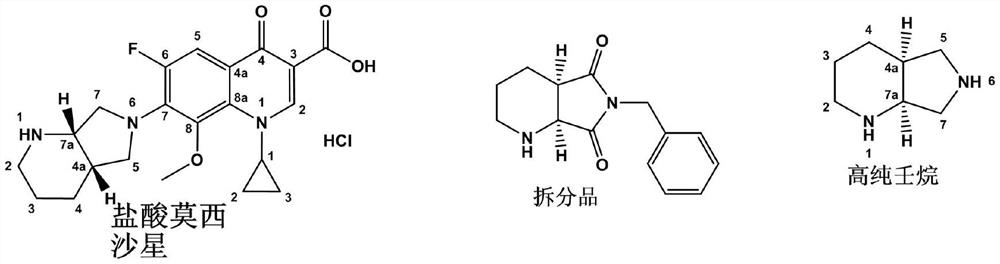
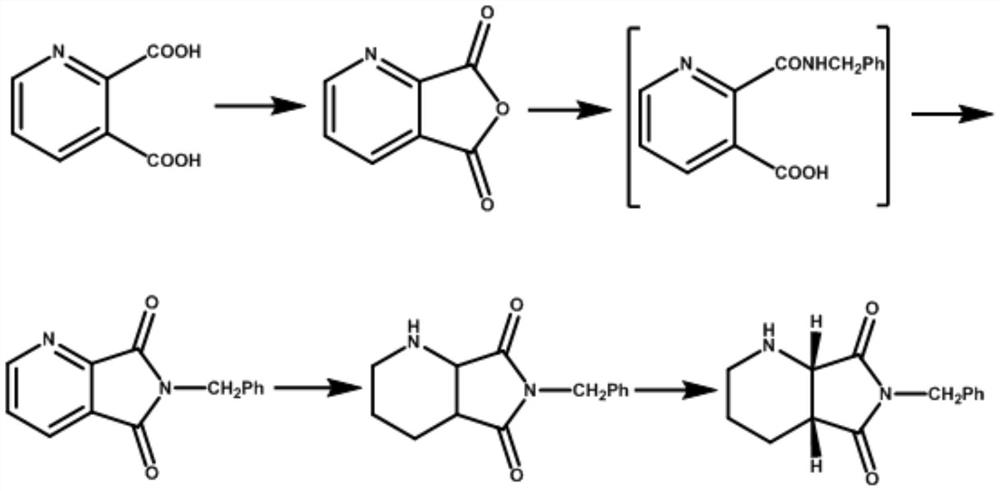
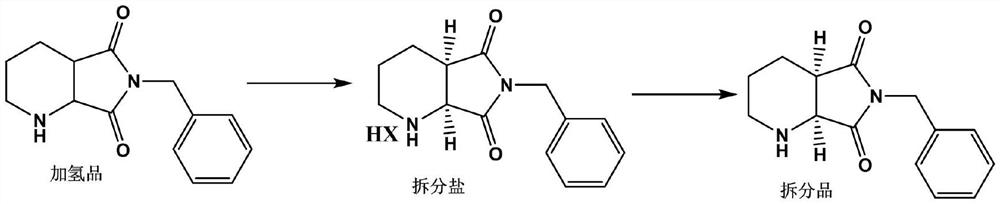
A kind of preparation method of compound a
A compound and mixed solvent technology, applied in the preparation of chiral intermediate side chain -2,8-diazabicyclo[4.3.0]nonane, in the field of preparation of pharmaceutical intermediates, can solve the problem of unstable resolving agent, Solve problems such as low yield and complicated process, achieve high industrial production value, reduce production cost, and achieve high chiral purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] A method of preparation of compound A, the compound A is (S, R) -8-benzyl-7,9-dioxidant-2,8-diazorane bicyclic ring [4.3.0] decane, its step include:
[0032] (1) Preparation of the split salt: dissolve the hydrogenate in a mixed solvent to add the splitter coolean acid, and the temperature is warmed to react, then cool the crystalline, filtrate, wash, dry the salt;
[0033] (2) Stripping the alkaloid: The splitting salt obtained by step (1) is extracted by alkali, organic solvent, and the solvent is evaporated, the EE value is greater than 98% (S, R) -8-benzyl-7, 9-dioxidine-2,8-diazorane bicyclic ring [4.3.0] decane.
[0034] The product (S, R) 8-benzyl-7,9-dioxo-2,8-diazine-7,9-dioxo-2,8-diaza-7,9-dioxo-2,8-diazine-7,9-dioxo-2,8-diazine-7,9-dioxo-2,8-bisz-7,9-dioxo-2,8-diazo-7,9-dioxane-proof Determination of Moshisha Cross of Moshisa Food and Drug Food and Drug in 2013.
Embodiment 1
[0036] (1) 50 g of a hydrogen product of 98% of the liquid phase purity was dissolved in a mixed solvent of 300 ml of ethanol, butanone and water in which the water obtained was 10%, and 20.19 g of citrison acid was added after dissolving, and heated to 50 ° C. 2 hours, then cooled to 20 degrees Celsius for 2 hours crystalline, filtered, filter cake was flush with a mixed solvent of the above ethanol, butanone and water, and the filter cake was dried to obtain a split salt 36.6 g;
[0037] (2) The split salt obtained by step (1) was added to 80 ml of purified water, 8.0 g of slicat, and then adding the pH 8-9 to add toluene 80 ml * 2 extraction, dried over anhydrous sodium sulfate and evaporated. Oil liquid is (S, R) -8-benzyl-7,9-dioxidine-2,8-diazoracy double ring [4.3.0] decane 21.5 g, yield 43%; EE value 99%.
Embodiment 2
[0039] (1) 50 g of the hydrogen 50g of the liquid phase purity was dissolved in 150 mL of methanol and water mixed solvent, wherein the water-based volume fraction was 5%, and 40.38 g of ancient dragon acid was added after dissolving, and the water was gradually warmed to 80 ° C for 1 hour. Then, then slowly cool to 30 degrees Celsius insulation for 1 hour. Filtration, the filter cake was flushed with 100 mL of the above methanol and the mixed solvent of water, and the filter cake was dried to obtain a split salt 38.1 g.
[0040] (2) The split salt obtained by step (1) was added 80 ml of purified water, 8.0 g of a piece of base, and then adding the pH 8-9 to 80 ml * 2 extraction, dried over anhydrous sodium sulfate and evaporated. Yellow oily liquid is (S, R) -8-benzyl-7,9-dioxo-2,8-diaza-bicyclic ring [4.3.0] decane 20.8 g, yield 41.6%; EE value 98.2% .
PUM
| Property | Measurement | Unit |
|---|---|---|
| chiral purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com