Application of composition to preparation of medicine for treating gastric cancer based on regulation and control of cancer suppression related genes
A technology of composition and application, which is applied in the field of preparing gastric cancer drugs based on the regulation of tumor suppressor-related genes, and can solve the problems of poor prognosis and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Moluotan is composed of lily, Poria cocos, Scrophulariaceae, black medicine, Alisma, Ophiopogon japonicus, Angelica, Atractylodes macrocephala, capillary root, white peony root, dendrobium, calamus chinensis, Chuanxiong, notoginseng, Burnet, Corydalis, Puhuang, chicken The pure Chinese medicine preparation composed of 18 medicinal materials such as Neijin has the functions of harmonizing the stomach and invigorating the spleen, dredging collaterals and relieving pain, strengthening the spleen and reducing swelling, and can significantly reduce the expression of EGF and EGFR in patients with CAG, and the actual clinical application effect is quite good. Moluodan was selected as the treatment for chronic gastritis and chronic atrophy by the "Consensus Opinions on Chronic Gastritis in China" (2017) formulated by the Digestive Disease Branch of the Chinese Medical Association, and the "Consensus Opinions on the Diagnosis and Treatment of Chronic Gastritis by TCM Experts" (201...
Embodiment 2
[0058] Example 2 Identification of substances with tumor suppressor function of Molotan.
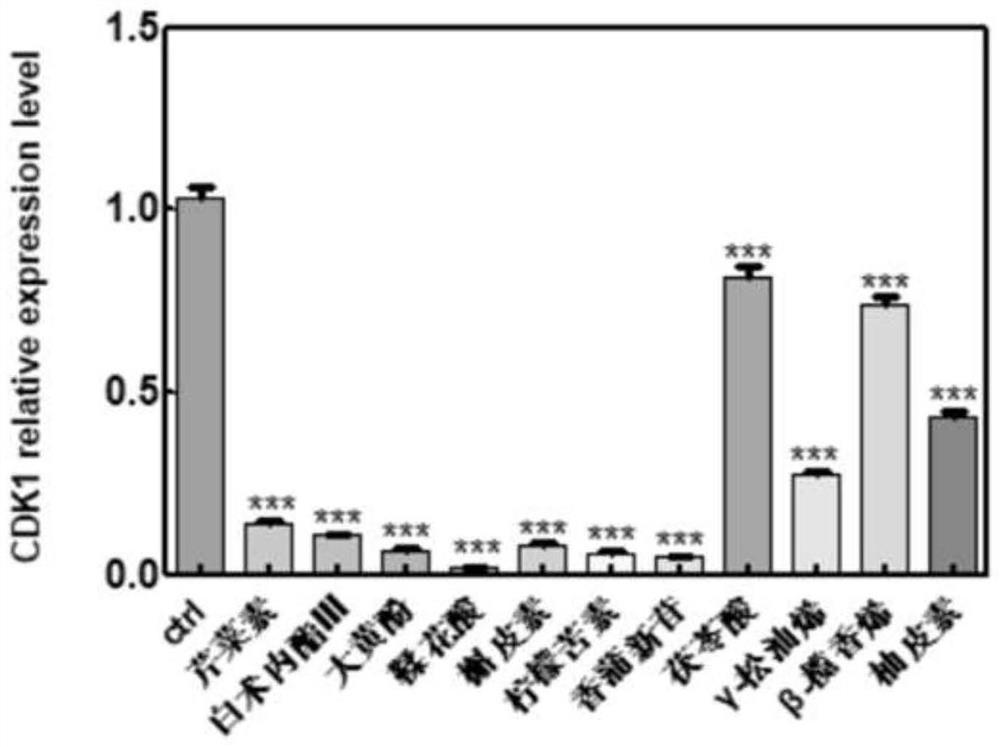
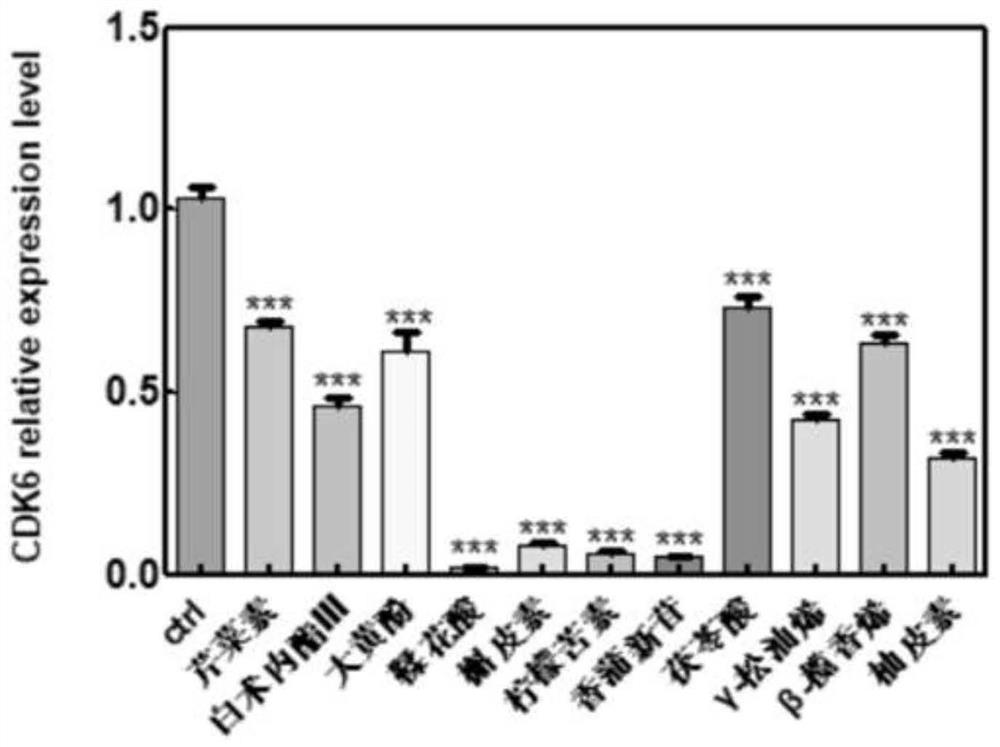
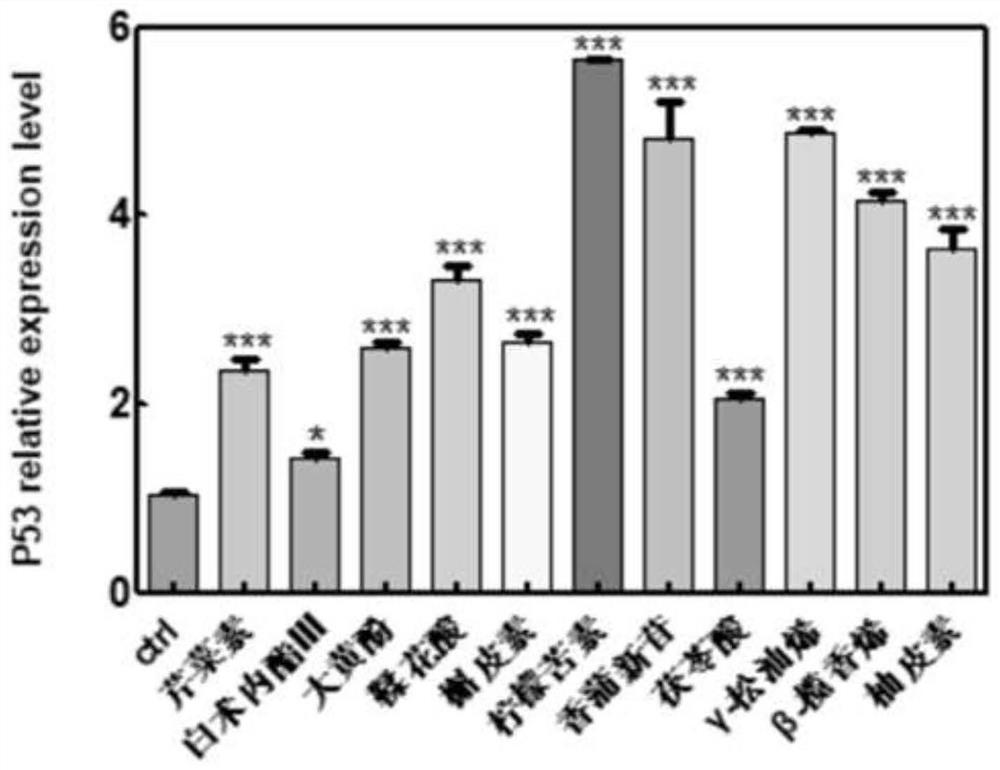
[0059] In this example, among the genes with significant changes in expression levels in the transcriptome analysis data, the changes in the transcripts of genes such as P53, TGF-β, CDK1, and CDK6 were used as marker genes for regulating tumor suppressors.
[0060] SGC-7901 cell line (preserved by our laboratory and purchased from ATCC cell bank) is used as the object of drug treatment. This cell has the characteristics of rapid reproduction and easy culture. The cultured SGC-7901 cells were seeded on a 6-well plate (50w cells were cultured in each well), and cultured in DMEM (10% FBS) medium until all adhered. The molodan candidate active components screened above were dissolved in DMSO, and applied to the SGC-7901 cell culture medium respectively, and the treated cells were collected after incubation for 24 hours. RNA was extracted according to the processing steps in the aforemention...
Embodiment 3
[0064] In this embodiment, apigenin, atractylodes lactone III, chrysophanol, ellagic acid, quercetin, limonin, cattail glycoside, pachymic acid, γ-terpinene, β-elemene and pomelo The 11 substances of corticosteroids were combined according to different molar ratios, and the advantages of promoting cell apoptosis under different combinations were verified.
[0065] SGC-7901 cell line (preserved by our laboratory and purchased from ATCC cell bank) is used as the object of drug treatment. This cell has the characteristics of rapid reproduction and easy culture.
[0066] Inoculate the cultured SGC-7901 cells on a 6-well plate (50w cells per well), culture them in DMEM (10% FBS) medium until they are all attached to the wall, and dissolve the 11 effective components screened above in DMSO , mix according to the following four combinations (molar concentration ratio):
[0067] Group A: Apigenin: Atractylodes lactone III: Chrysophanol: Ellagic acid: Quercetin = 4:10:1:6:8;
[0068]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com