Vaccine containing capsular polysaccharide of type 5 streptococcus pneumoniae and preparation method thereof
A technology of Streptococcus pneumoniae and capsular polysaccharide, which can be used in medical preparations containing active ingredients, medical preparations without active ingredients, and antibacterial drugs, which can solve the risk of protein immunogenicity and loss of specific epitopes. , antigen destruction, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
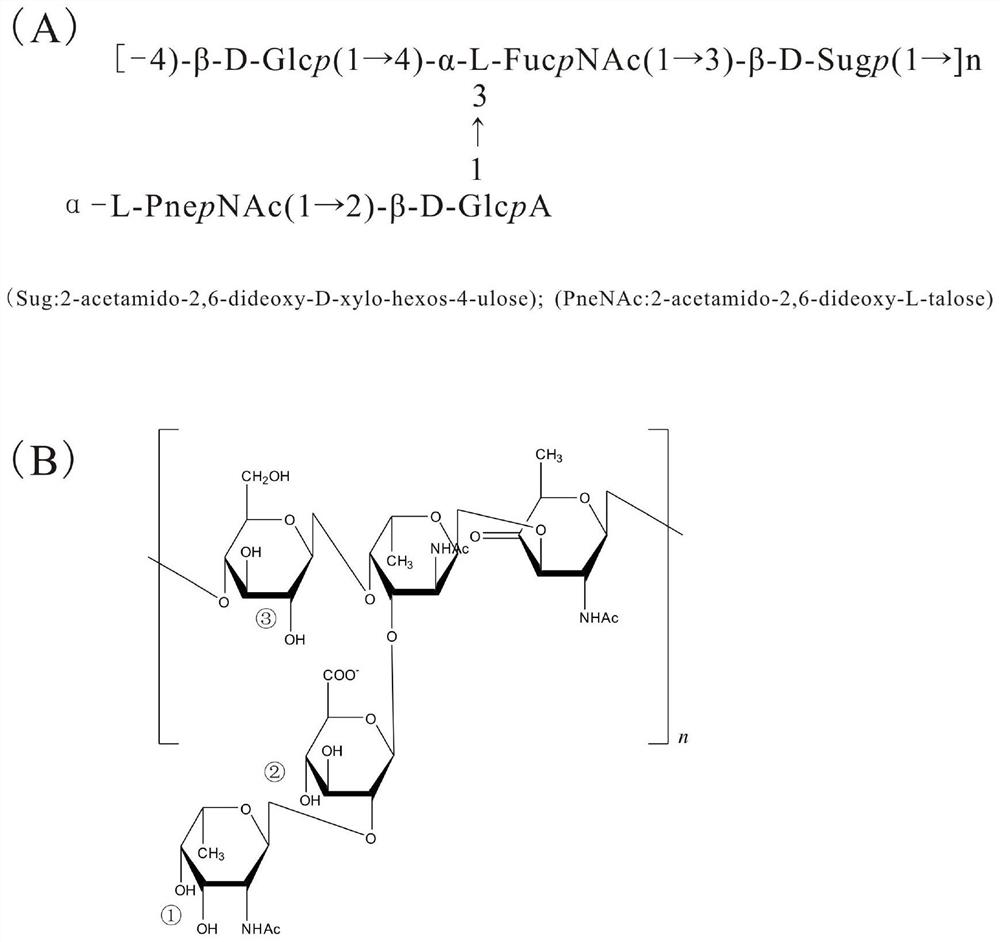
[0072] Example 1 Preparation of Streptococcus pneumoniae serotype 5 capsular polysaccharide-protein conjugate
[0073] Serotype 5 capsular polysaccharides can be obtained directly from bacteria using isolation methods known to those skilled in the art. Serotype 5 pneumococcus (Streptococcus pneumoniae) strains were obtained from China National Institutes for Food and Drug Control. The above strains were subcultured to build a library. The strains were inoculated in a liquid medium and cultured at 35±2°C. In the late logarithmic growth period, sodium deoxycholate was added to lyse the bacteria and release capsular polysaccharides, centrifuge, and collect the supernatant. Concentrate the supernatant 3-10 times by ultrafiltration with ultrafiltration membrane packs with a molecular weight cut-off of 100 or 300 kDa. Use a phosphate buffer solution with a pH of about 7.0 to replace the ultrafiltrate by ultrafiltration, then add absolute ethanol to make the final concentration of ...
Embodiment 2
[0122] Example 2 Evaluation of Streptococcus pneumoniae serotype 5 capsular polysaccharide-protein conjugates
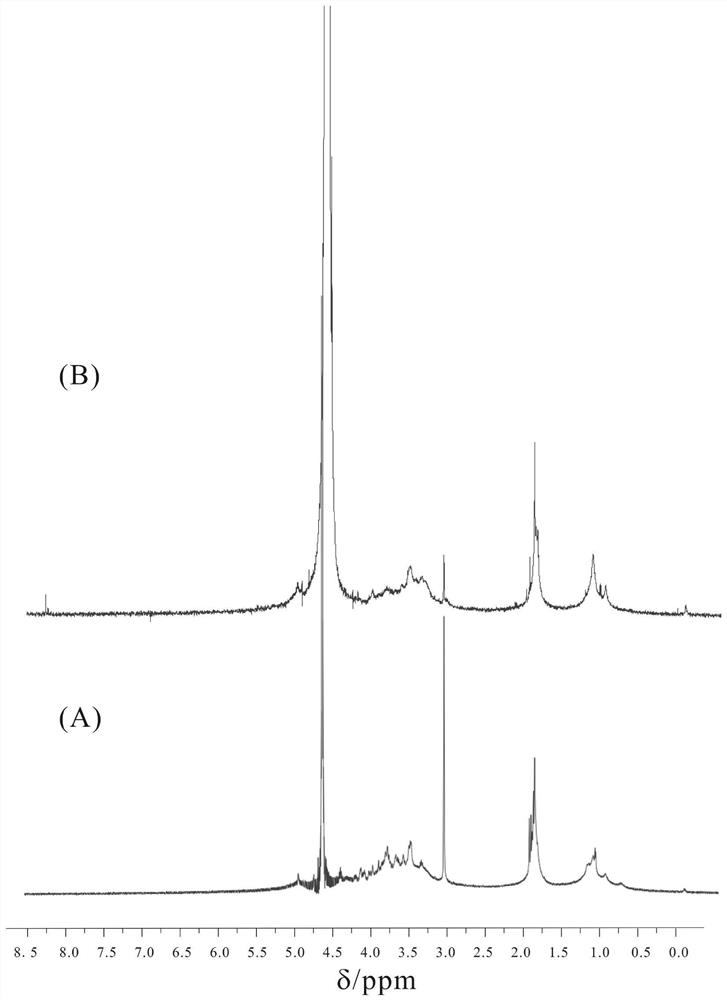
[0123] Determination of the structural integrity of capsular polysaccharides: using the literature method (Talaga P, Vialle S, Moreau M. Development of a high-performance anion-exchange chromatography with pulsed-amperometric detection based quantification assay for pneumococcal polysaccharides and conjugates[J].Vaccine,2002, 20(19):2474-2484.), using ion chromatography to analyze the content of PnepNAc sugar in activated polysaccharides and bound polysaccharides, taking Fuc as an internal reference, and calculating the structural integrity, the results are shown in Table 7.
[0124] Evaluation of immunogenicity of capsular polysaccharide-protein conjugates: BALB / c mice of 18-22 g were immunized, the polysaccharide content of each immune substance was 2 μg / ml, and 10 mice were immunized in each group. Two injections of intraperitoneal immunization on days 0 and 14, 0...
Embodiment 3
[0130] Example 3 Preparation of multivalent pneumococcal capsular polysaccharide conjugate vaccine
[0131] Mix the type 5 monovalent conjugates prepared above with 1, 3, 4, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 23F monovalent conjugates, according to the final concentration of polysaccharide 4μg / ml, 6B The conjugates were mixed with each conjugate according to the final polysaccharide concentration of 8 μg / ml, and mixed with physiological saline as a solvent to prepare a 13-valent pneumonia polysaccharide conjugate vaccine preparation for injection. The 13-valent pneumococcal capsular polysaccharide conjugate vaccine prepared above is made into a freeze-dried dosage form by low-temperature drying, and reconstituted with physiological saline before use.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com