Adsorbent for removing nitric oxide in fluid as well as preparation method and application of adsorbent
A technology of nitrogen oxides and adsorbents, applied in the field of adsorbents for removing nitrogen oxides in fluids, can solve the problem of low removal efficiency of nitrogen oxides, achieve strong selective adsorption of nitrogen oxides, good adsorption Performance, the effect of controlling the crystal form
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
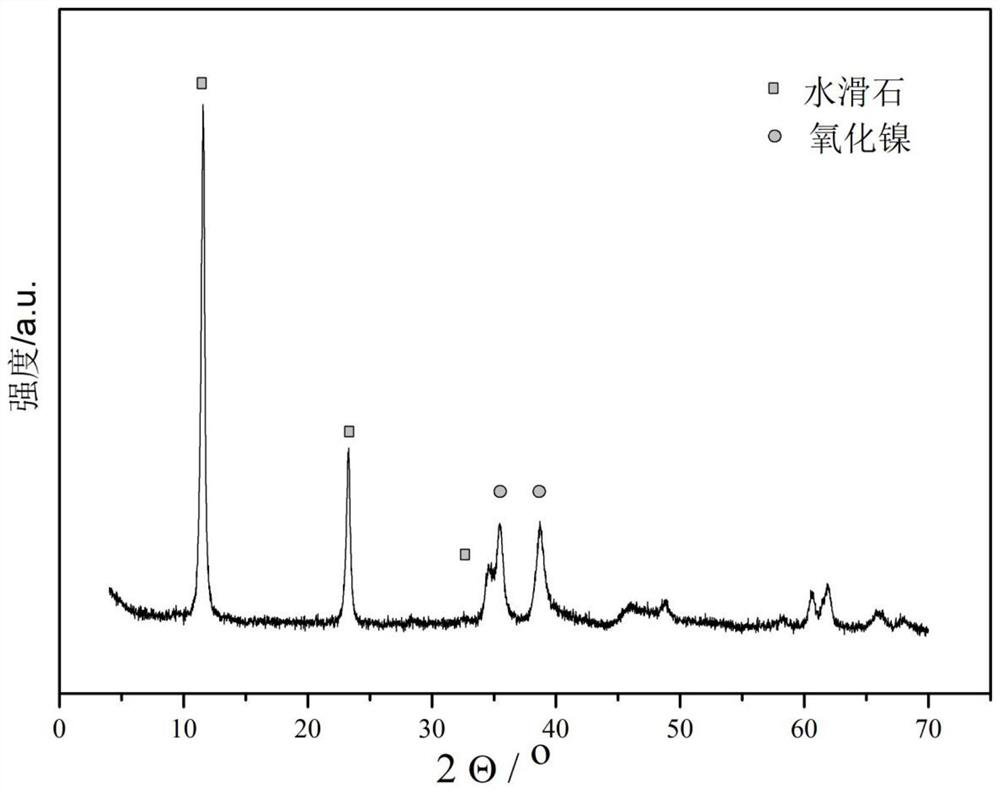
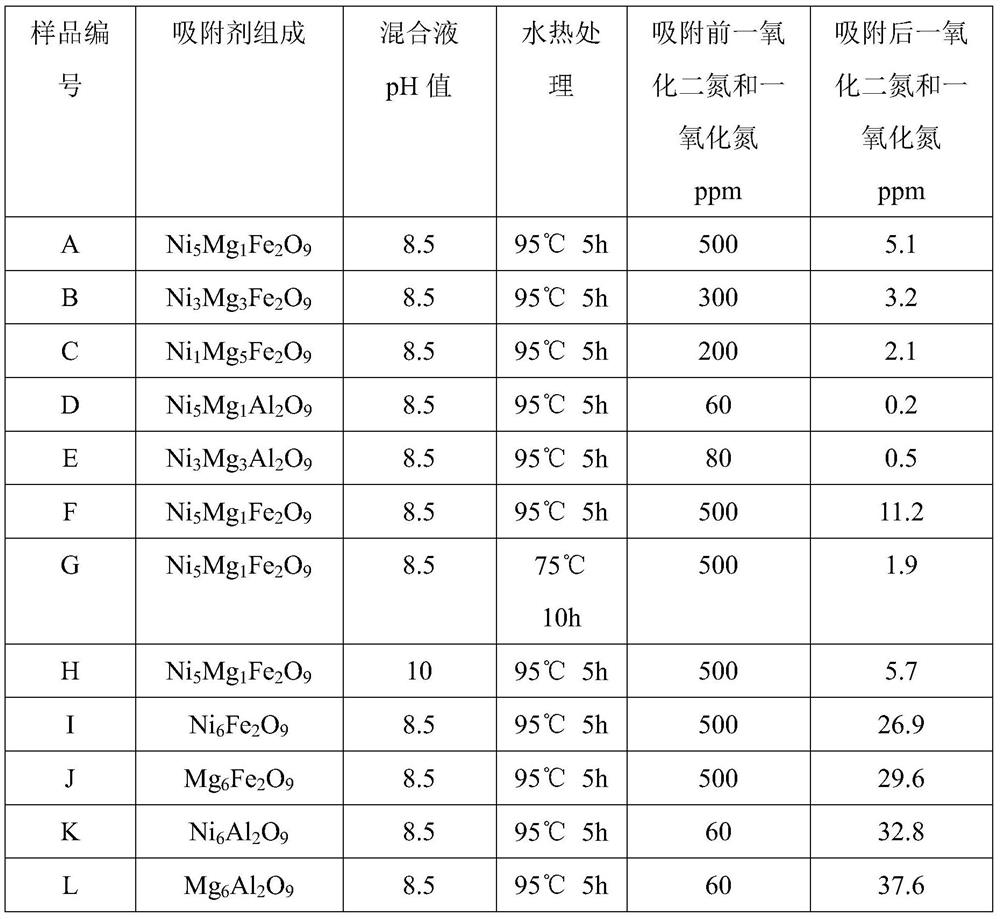
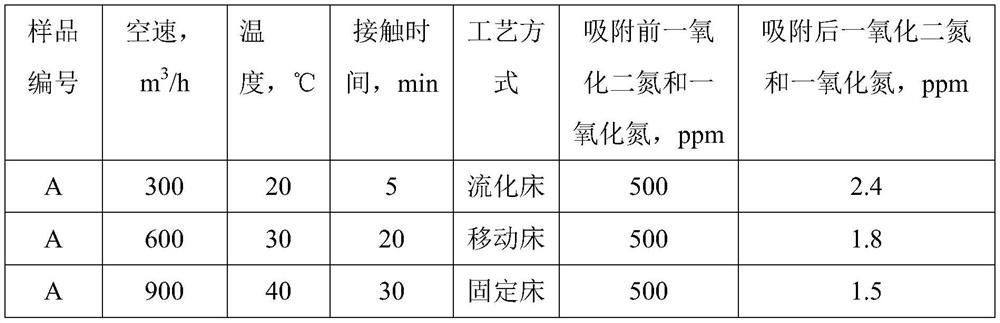
[0044] Preparation of Solution A: Dissolve nickel nitrate, magnesium nitrate and ferric nitrate in deionized water at room temperature in a molar ratio of 5:1:2, stir well until clear, add 300mL polyethylene glycol solution with a concentration of 5wt%, and stir 10mins. Preparation of solution B: Dissolve sodium hydroxide in deionized water at room temperature and stir until clear. Solution B was rapidly added dropwise into solution A until the pH of the mixed solution reached 8.5, and stirring was continued at room temperature for 2 h to obtain mixture C. The mixture C was placed in a crystallization tank and subjected to hydrothermal treatment at 95° C. for 5 hours. The product was filtered, washed with deionized water, dried in an oven at 120°C for 10 hours, and calcined in a muffle furnace at 450°C for 4 hours. The product obtained was the adsorbent product A.
[0045] Add 20g of the adsorbent A prepared above to 1L aqueous solution containing 500ppm nitric oxide and nit...
Embodiment 2
[0047] Preparation of solution A: Dissolve nickel nitrate, magnesium nitrate and ferric nitrate in deionized water at room temperature at a molar ratio of 3:3:2, stir well until clear, add 300mL of a solution with a concentration of 5wt% polyethylene glycol, and disperse 10mins. Preparation of solution B: Dissolve sodium hydroxide in deionized water at room temperature and stir until clear. Solution B was rapidly added dropwise into solution A until the pH of the mixed solution reached 8.5, and stirring was continued at room temperature for 2 h to obtain mixture C. The mixture C was placed in a crystallization tank and subjected to hydrothermal treatment at 95° C. for 5 hours. The product was filtered, washed with deionized water, dried in an oven at 120°C for 10 hours, and calcined in a muffle furnace at 550°C for 2 hours. The product obtained was the adsorbent product B.
[0048] Add 20g of the adsorbent B prepared above to 1L aqueous solution containing 300ppm nitrous oxi...
Embodiment 3
[0050] Preparation of solution A: Dissolve nickel nitrate, magnesium nitrate and ferric nitrate in deionized water at room temperature at a molar ratio of 1:5:2, stir well until clear, add 100mL of 2wt% urea solution, and disperse for 10mins. Preparation of solution B: Dissolve sodium hydroxide in deionized water at room temperature and stir until clear. Solution B was rapidly added dropwise into solution A until the pH of the mixed solution reached 8.5, and stirring was continued at room temperature for 2 h to obtain mixture C. The mixture C was placed in a crystallization tank and subjected to hydrothermal treatment at 95° C. for 5 hours. The product was filtered, washed with deionized water, dried in an oven at 120°C for 10 hours, and calcined in a muffle furnace at 500°C for 4 hours. The obtained product was adsorbent product C.
[0051] Add 20g of the above-prepared adsorbent C to 1L of aqueous solution containing 200ppm nitrous oxide and nitric oxide, stir fully at room...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com