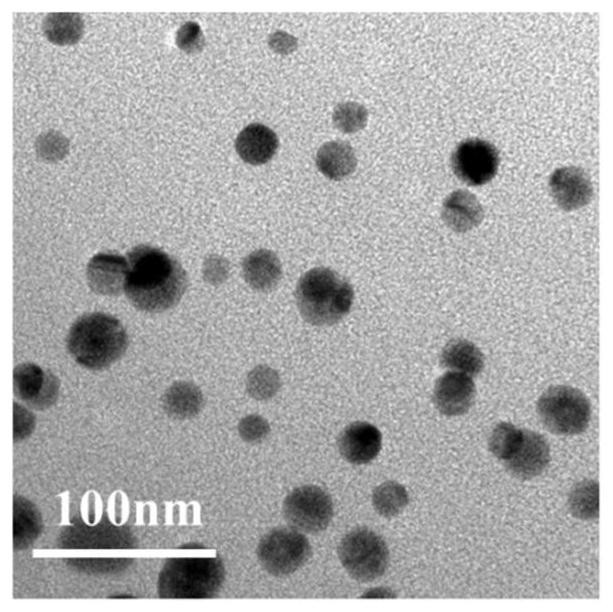
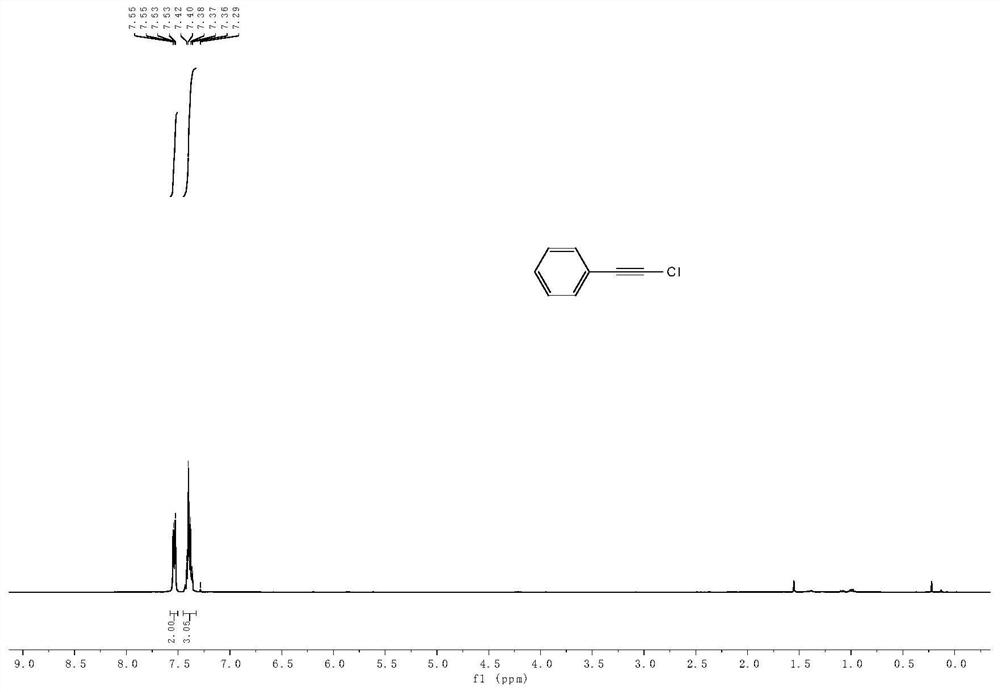
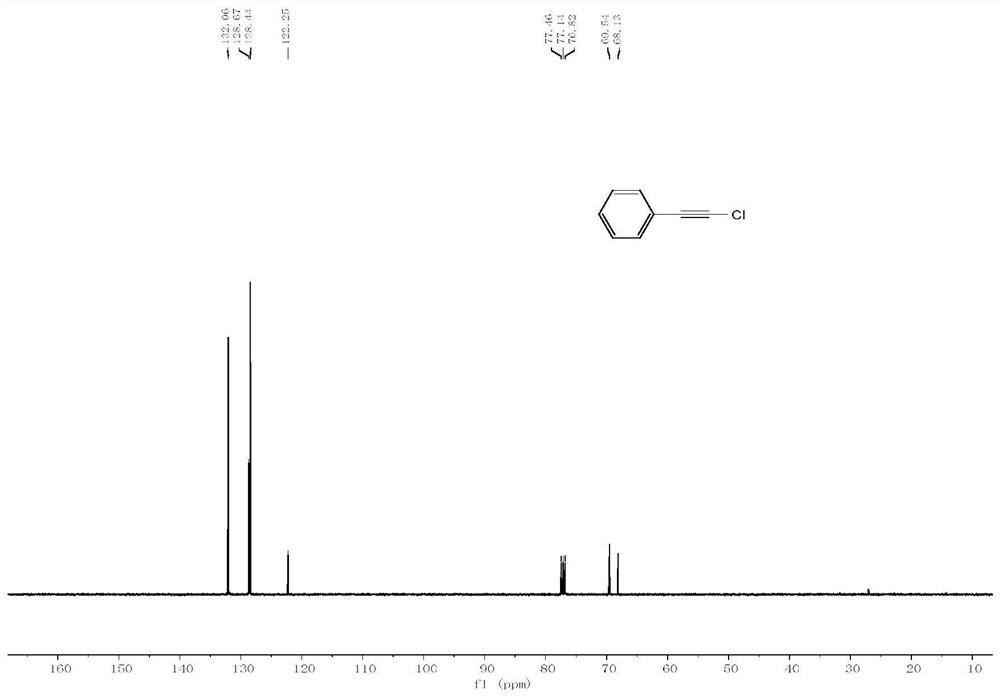
Method for preparing 1-halogenated alkyne under catalysis of heterogeneous Ag catalyst at room temperature
A catalytic preparation and catalyst technology, applied in the field of chemistry, can solve the problems of complex reaction process, low product selectivity, catalyst cannot be recycled and reused, etc., and achieve the effect of good tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The compound N-chlorosuccinimide (0.45 mmol), potassium acetate (0.3 mmol), 20 mg Ag@C-2 (12.1 wt% silver content) catalyst, acetonitrile (3 mL) and phenylacetylene (0.3 mmol) were sequentially added to a 10 mL brown vial and reacted at 25°C for 6 hours. The reaction product was confirmed by NMR analysis that the main product was indeed 1-haloalkyne. Using naphthalene as an internal standard, the yield of 1-chloroalkynes was 93% through quantitative analysis by gas chromatography.
Embodiment 2
[0027] It is basically the same as Example 1, the difference is: 39 mg Ag@C-1 (silver content is 6.2wt%) catalyst is used instead of Ag@C-2 in Example 1, the test result is: this example obtained 1 - The yield of chloroalkynes was 91%.
Embodiment 3
[0029] It is basically the same as Example 1, the difference is: 16 mg Ag@C-1 (silver content is 15.5wt%) catalyst is used instead of Ag@C-2 in Example 1, the test result is: this example obtained 1 - The yield of chloroalkynes was 84%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com