Degradable polyester rubber as well as preparation method and application thereof
A technology for degrading polyester and rubber, applied in chemical instruments and methods, other chemical processes, paints containing biocides, etc., can solve problems such as oil and gas mining construction failures, deterioration of material mechanical properties, and easy tearing, and achieve excellent results Processability and vulcanization characteristics, adjustable degradation rate, wide range of application effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
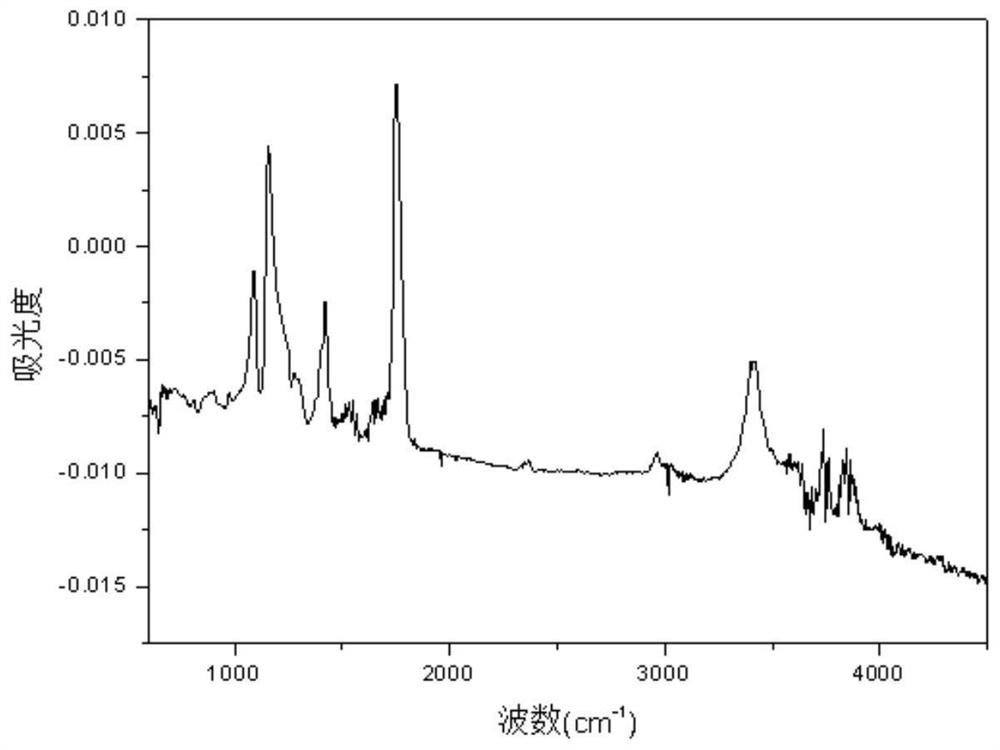
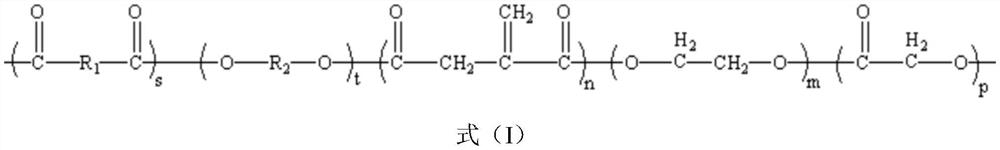
Image
Examples
preparation example Construction
[0028] An aspect of the embodiment of the present invention provides a kind of preparation method of degradable polyester rubber, it comprises:
[0029] Under vacuum conditions, the first mixed reaction system comprising dimer acid, polyglycol, esterification catalyst and polycondensation catalyst undergoes a primary esterification reaction;
[0030] Adding itaconic acid, ethylene glycol and a degradation regulator to the first mixed system to form a second mixed reaction system, and a secondary esterification reaction at 130-150°C for 2-4 hours;
[0031] And, the vacuum degree of the reaction system obtained after the secondary esterification reaction is reduced to less than 200 Pa, and then the polycondensation reaction is carried out at 200-230° C. for 4-6 hours to obtain the degradable polyester rubber.
[0032] In some more specific embodiments, the preparation method includes: under the condition of a vacuum degree of 500-1000Pa, making the first mixed reaction system co...
Embodiment 1
[0076] (1) primary esterification: with 0.1mol dimer acid, 0.2mol triethylene glycol, 0.001mol tetraethylammonium bromide (consumption is 1% of dimer acid mol amount), 1.734g titanium glycolate (consumption 1% of the total mass of dimer acid and itaconic acid) into the reactor together, start stirring 100r / min, under high vacuum 1000Pa (absolute pressure), reaction temperature 120 ℃, esterification reaction 1h, then heat up To 140°C, continue to react for 1 hour to complete primary esterification;
[0077] (2) Secondary esterification: continue to put 0.9mol itaconic acid, 1.8mol ethylene glycol, 0.05mol methyl glycolate into the reaction kettle, under the conditions of 130°C normal pressure, nitrogen protection, stirring rate 80r / min Lower reaction 4h;
[0078] (3) Polycondensation: start stirring at 100r / min, gradually increase the vacuum degree to the absolute pressure of reaction 200Pa, react at 200°C for 6h, and press out with nitrogen to obtain degradable polyester rubb...
Embodiment 2
[0081] (1) primary esterification: with 0.2mol dimer acid, 0.3mol triethylene glycol, 0.001mol tetrabutylammonium bromide (consumption is 0.5% of dimer acid mol amount), 1.084g tetrabutyl titanate ( The amount used is 0.5% of the total mass of dimer acid and itaconic acid) into the reactor together, the stirring rate is turned on at 5r / min, under the conditions of high vacuum 500Pa (absolute pressure) and reaction temperature 130°C, the esterification reaction is carried out for 2h, Then the temperature was raised to 145° C., and the reaction was continued for 2 hours to complete the primary esterification;
[0082] (2) Secondary esterification: continue to put 0.8mol itaconic acid, 2.4mol ethylene glycol, 0.1mol ethyl glycolate into the reaction kettle, under the conditions of 150°C normal pressure, nitrogen protection, stirring rate 5r / min Lower reaction 2h;
[0083] (3) Polycondensation: Start stirring for 5r / min, gradually increase the vacuum degree to the absolute reacti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com