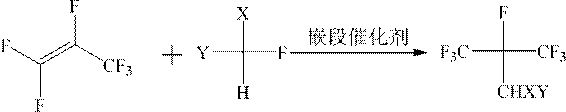
Method for synthesizing fluorinated isobutene with hexafluoropropylene as starting material
A technology for fluoroisobutene and hexafluoropropene, which is applied in the field of gas-phase reaction for preparing fluoroisobutene, can solve the problems of low synthesis efficiency, prone to detonation, low initial reaction temperature, etc., and achieves high yield, long service life and high activity. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
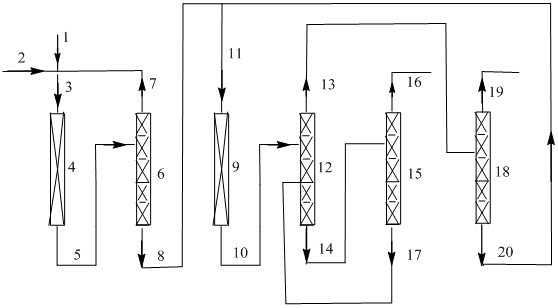
Image
Examples
preparation example Construction
[0066] (1) Preparation of dehydrofluorination catalyst: Dissolve chromium trichloride in water, add concentrated ammonia water dropwise for precipitation, adjust the pH value to 7.5, then age for 24 hours, wash with water, filter, and dry in an oven at 120°C for 15 hours. The obtained solid was pulverized and pressed into tablets to obtain a catalyst precursor. Put 10mL of the catalyst precursor into a Monel tubular reactor with an inner diameter of 1 / 2 inch and a length of 30cm, and bake it at 350°C for 12 hours with nitrogen gas , nitrogen space velocity is 200h -1 , and then lower the temperature to 300°C, and at the same time pass a mixed gas composed of hydrogen fluoride and nitrogen with a mass ratio of 1:2, and the total space velocity of the gas is 220h -1 , activate for 12-24 hours, stop the above mixed gas, and make CrF 3 ; The activation time can be shortened, such as activation for 6-10 hours, to obtain chromium oxide fluoride CrO x f y (2x+y=3, and both x and y...
Embodiment 1
[0071] Prepare the block catalyst SbF in a tubular reactor made of Incon alloy with an inner diameter of 1 / 2 inch and a length of 30 cm 5 / MgF 2 (Mass percentages are 20%:80%) 10mL. The reaction conditions are as follows: the reaction temperature is 50° C., the mass ratio of hexafluoropropylene and trifluoromethane is 1:5, the contact time is 100 s, and the reaction pressure is 0.1 MPa. After running for 10 hours, the reaction product was washed with water, alkali and dried, and the organic phase was taken for GC analysis. The reaction results are: the conversion rate of hexafluoropropene is 65.3%, and the selectivity of 2-(difluoromethyl)-1,1,1,2,3,3,3-heptafluoropropane is 99.8%.
Embodiment 2
[0073] The prepared block catalyst SbF is filled in a tubular reactor made of Incon alloy with an inner diameter of 1 / 2 inch and a length of 30 cm. 5 / MgF 2 (Mass percentages are 20%:80%) 10mL. The reaction conditions are as follows: the reaction temperature is 100° C., the mass ratio of hexafluoropropylene and trifluoromethane is 1:5, the contact time is 100 s, and the reaction pressure is 0.1 MPa. After running for 10 hours, the reaction product was washed with water, alkali and dried, and the organic phase was taken for GC analysis. The reaction results are: the conversion rate of hexafluoropropene is 84.7%, and the selectivity of 2-(difluoromethyl)-1,1,1,2,3,3,3-heptafluoropropane is 99.6%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com