Stable polypeptide protein targeted inhibitor and application thereof
A polypeptide and protein target technology, applied in the field of bioengineering, can solve problems such as poor breast cancer effect, achieve significant technological progress, inhibit growth, and broaden the application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
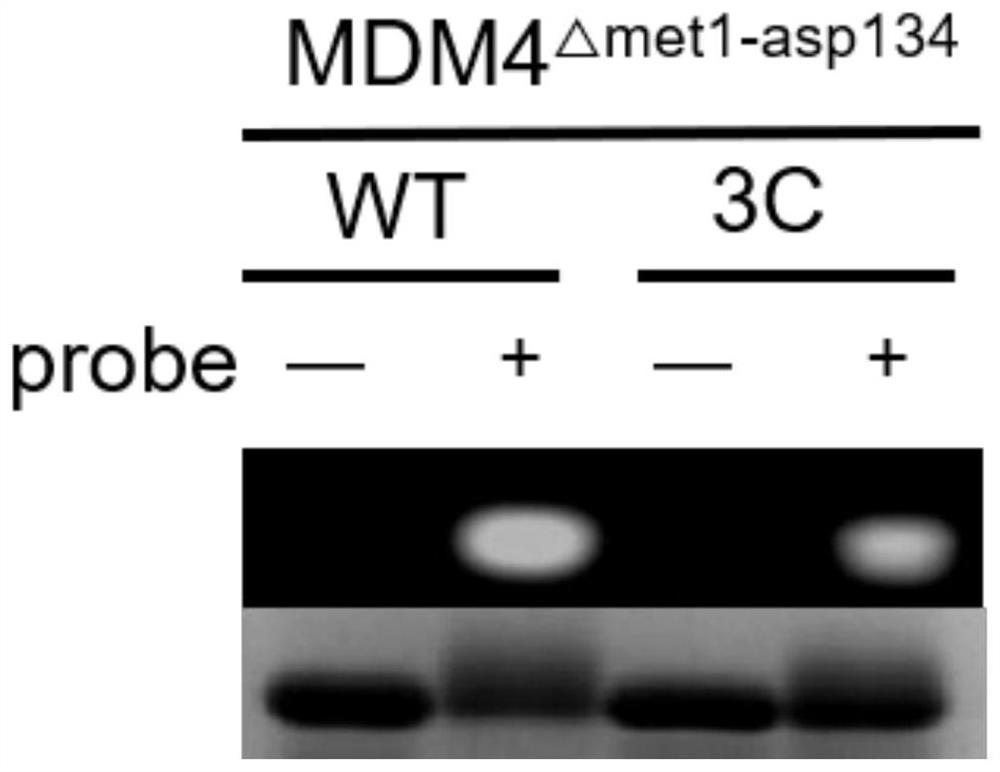
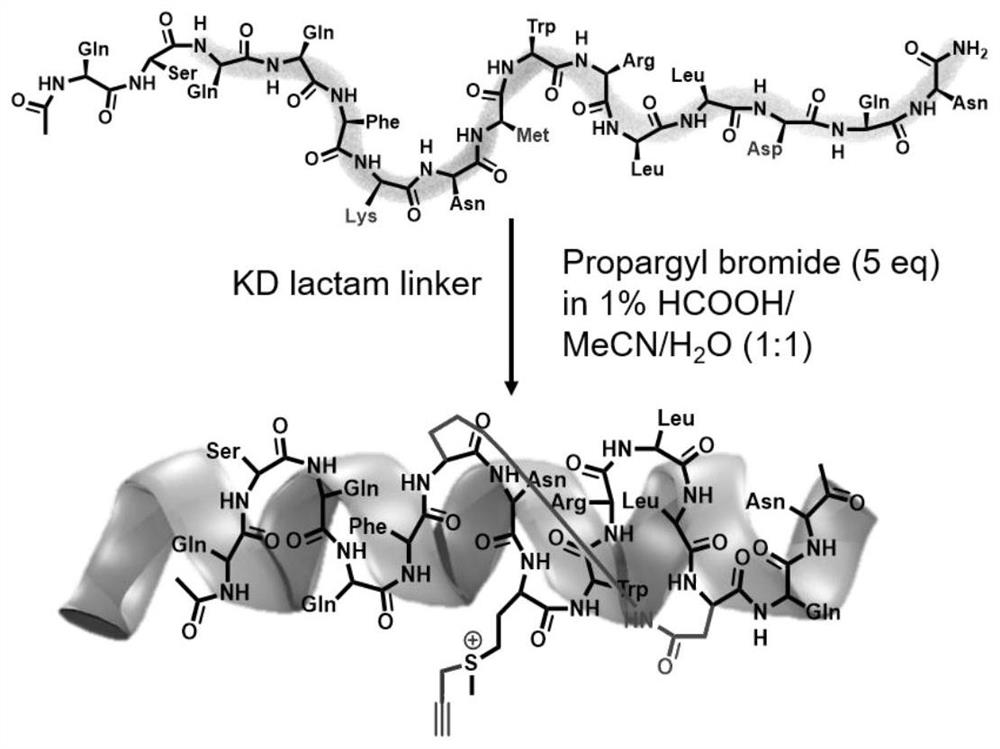
[0034]In order to better carry out the following research, the design of the control peptide is necessary. As shown in Table 1, we designed a series of control peptides. The present invention selects the SAH-p53-8 sequence (Ac-Q-S-Q-Q-T-F-*-N-X-W-R-L-L-#-Q-N-NH2) as the original sequence, and replaces it with methionine at the X site. And combined with the research strategy of Professor Wang Lei's research group (L. Wang, Chem. Commun., 2016, 52, 5140), they replaced the 22-position leucine in the SAH-p53-8 sequence with an aromatic group-containing Sulfonyl fluoride compound, which increases the inhibitory effect of SAH-p53-8 by 10 times. In the present invention, lysine and aspartic acid are used for side chain closure at position i and position i+7, thereby further stabilizing the polypeptide. The synthesized polypeptide (1equiv.) was reacted with propyne bromide reagent (5equiv.) under acidic conditions for 12 hours to construct the polypeptides in Table 1. The present ...
Embodiment 2
[0037] Preparation and separation and purification steps of the polypeptide of embodiment 2:
[0038] According to the amino acid sequence of solid-phase synthesis of polypeptides, the core steps for preparing the above-mentioned stable polypeptides are as follows (taking Peptide-2 as an example):
[0039]
[0040] The specific operation steps are:
[0041] (1) Polypeptide solid-phase synthesis: Weigh 100 mg Rink amide MBHA resin into a 10 ml peptide tube, add dichloromethane (DCM), and swell with nitrogen gas for 30 min. Add 50% (v / v) morpholine in N,N-dimethylformamide (DMF) solution, blow nitrogen gas for 30 minutes, and remove the Fmoc protecting group. After washing the resin 6 times alternately with DMF and DCM, the prepared (1) Fmoc-Asn-OH (5eq, 0.4M, DMF) solution, 6-chlorobenzotriazole-1,1,3,3-tetramethyl Urea hexafluorophosphate (HCTU) (5eq, 0.38M, DMF) solution and N,N-diisopropylethylamine (DIPEA) (10eq) were mixed well, then added to the resin and blown with ...
Embodiment 3
[0047] The experiment of embodiment 3 sulfonium salt-alkyne and lysine reaction
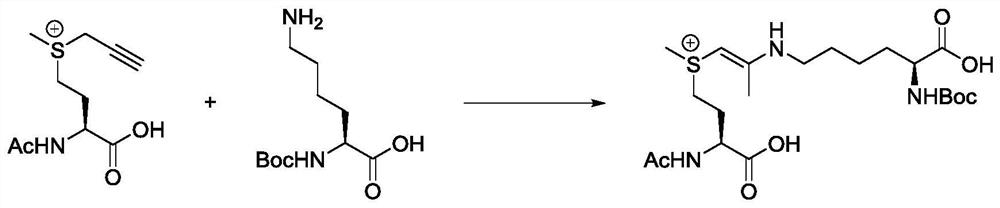
[0048] The present invention designs a model reaction, selects propargyl dimethyl sulfonium salt and Boc-Lys-OH as substrates to react in water for 12 hours, and then purifies by HPLC to obtain the target product. The present invention has carried out careful nuclear magnetic resonance (NMR) experiments, including 1H NMR, 13C{1H} NMR, heteronuclear single quantum correlation (HSQC), heteronuclear multiple bond correlation (HMBC) and total correlation spectroscopy (TOCSY), It can be confirmed that propargyl sulfonium salts can react with amino groups on lysine (such as Figure 4 ). Figure 4 A: 1H NMR: 1 H NMR (400MHz DMSO-d6) δ7.85–7.32(m,1H),6.29(d,J=7.5Hz,1H),4.39(s,1H),3.90–3.57(m,1H),2.83(s ,7H),2.05(s,3H),1.34(m,17H); Figure 4 B: 13 C{ 1 H}NMR: 13 C{ 1 H}NMR(400MHz DMSO-d6)δ174.7,158.6,158.5,158.2,155.3,118.8,115.8,77.6,69.1,54.6,52.0,44.9,42.9,31.9,31.5,28.3,27.1,23.1,18.1,8.8,7.7 ;...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com