Composition for improving scar hyperplasia and hyperpigmentation in skin repair process
A technology for improving skin and pigmentation, applied in skin diseases, drug combinations, pharmaceutical formulations, etc., can solve problems such as convenient drug application and fast drug absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
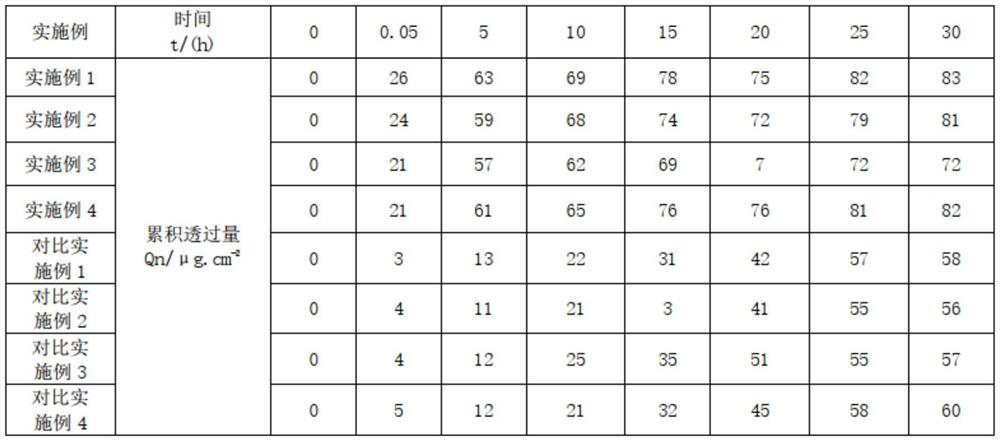
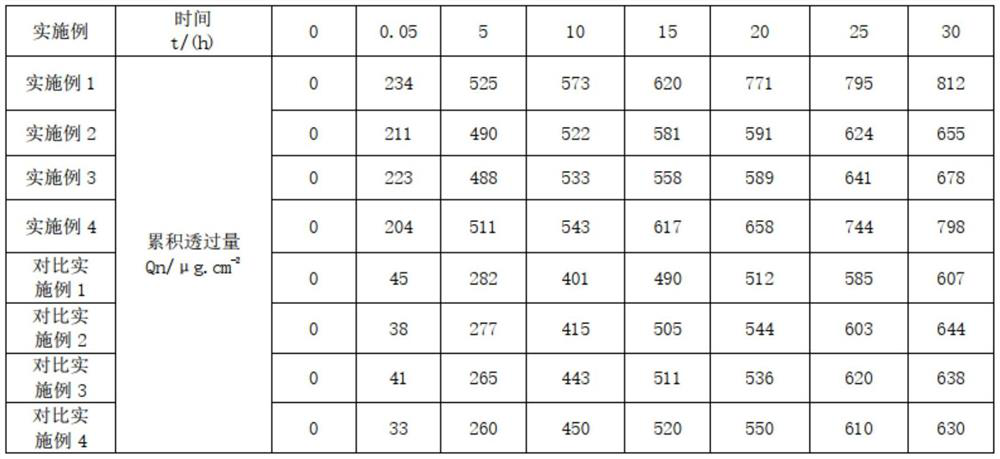
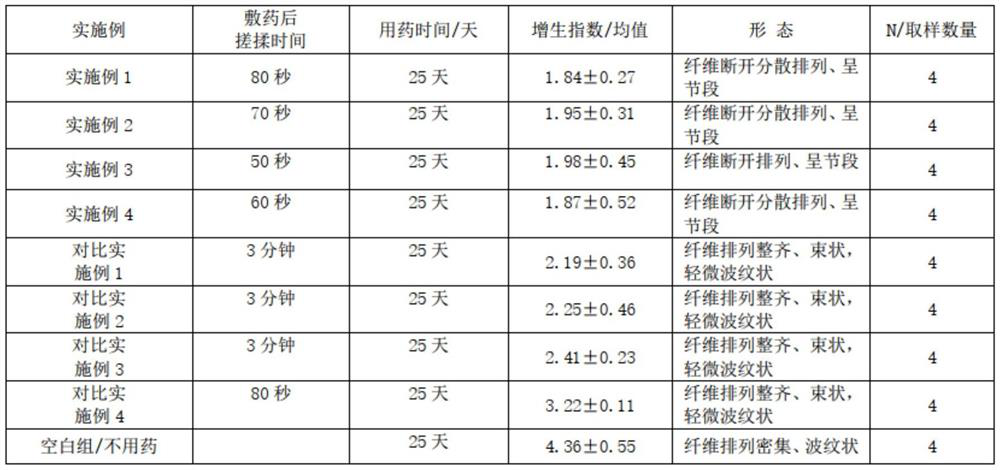
Examples
Embodiment 1
[0025] A prescription for improving scar hyperplasia and pigmentation in the process of skin repair. Its raw materials are 1.5 parts of asiaticoside, 0.5 parts of heparin sodium, 1 part of allantoin, 0.05 parts of Clostridium histolyticum collagenase, 78.5 parts of water, 3 parts of β-glucan, (composition of 1 part of glycerin, 1 part of propylene glycol, 0.5 parts of glycerin polymethacrylate and 0.5 parts of acrylic (ester) copolymer) 3 parts, acryloyl dimethyl 2 parts of ammonium taurate / VP copolymer, 0.2 parts of xanthan gum, 0.04 parts of disodium EDTA, 1 part of lecithin, 0.2 parts of centipede extract, 0.3 parts of toad extract, 1 part of ceramide, (1,2 - 0.3 parts of hexanediol, 0.5 parts of caprylyl hydroxyguanidine, 0.4 parts of 1,3-propanediol) 1.2 parts, 4.5 parts of silicone, 4 parts of 1,2-pentanediol, 0.5 parts of panthenol and lactic acid Bacteria consists of 2 parts.
[0026] The steps of preparing the gel medicine by formula in the present embodiment are as ...
Embodiment 2
[0034] A prescription for improving scar hyperplasia and pigmentation in the process of skin repair. Its raw materials are 2 parts of asiaticoside, 0.6 parts of heparin sodium, 1.5 parts of allantoin, 0.5 parts of Clostridium histolyticum collagenase, 72 parts of water, 3.5 parts of β-glucan, 5 parts of glycerin polymethacrylate, 3 parts of ammonium acryloyldimethyl taurate / VP copolymer, 0.3 parts of xanthan gum, 0.06 parts of disodium EDTA, eggs 3 parts of phospholipids, 0.3 parts of centipede extract, 0.4 parts of toad extract, 3 parts of ceramide, 4.8 parts of silicone.
[0035] The steps of preparing the gel medicine by formula in the present embodiment are as follows:
[0036] Step 1, heat the water to 90-100°C, keep it for 30 minutes, add EDTAA disodium after cooling to 30-35°C and stir evenly until no powder aggregates condense to obtain phase B. The vacuum degree during the stirring process is ≦-0.06MPa, Stirring speed is 50rpm;
[0037] Step 2: Under vacuum conditio...
Embodiment 3
[0043] A prescription for improving scar hyperplasia and pigmentation in the process of skin repair. Its raw materials are 2 parts of asiaticoside, 0.6 parts of heparin sodium, 1.5 parts of allantoin, 0.5 parts of Clostridium histolyticum collagenase, 70 parts of water, 3.5 parts of β-glucan, 5 parts of glycerin polymethacrylate, 3 parts of ammonium acryloyldimethyl taurate / VP copolymer, 0.3 parts of xanthan gum, 0.06 parts of disodium EDTA, nerve Composed of 3 parts of amide, 3 parts of lecithin, and 4.3 parts of silicone.
[0044] The steps of preparing the gel medicine by formula in the present embodiment are as follows:
[0045] Step 1, heat the water to 90-100°C, keep it for 30 minutes, add EDTAA disodium after cooling to 30-35°C and stir evenly until no powder aggregates condense to obtain phase B. The vacuum degree during the stirring process is ≦-0.06MPa, Stirring speed is 50rpm;
[0046] Step 2: Under vacuum conditions, mix 3 parts of ammonium acryloyldimethyltaurat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com