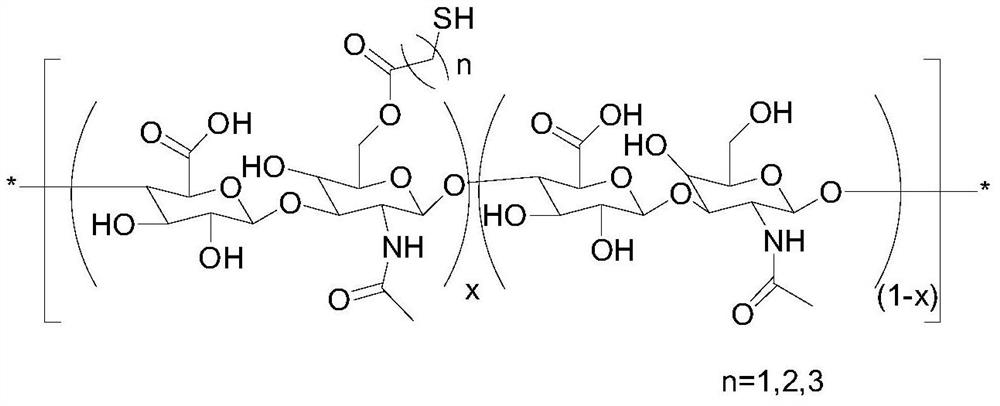
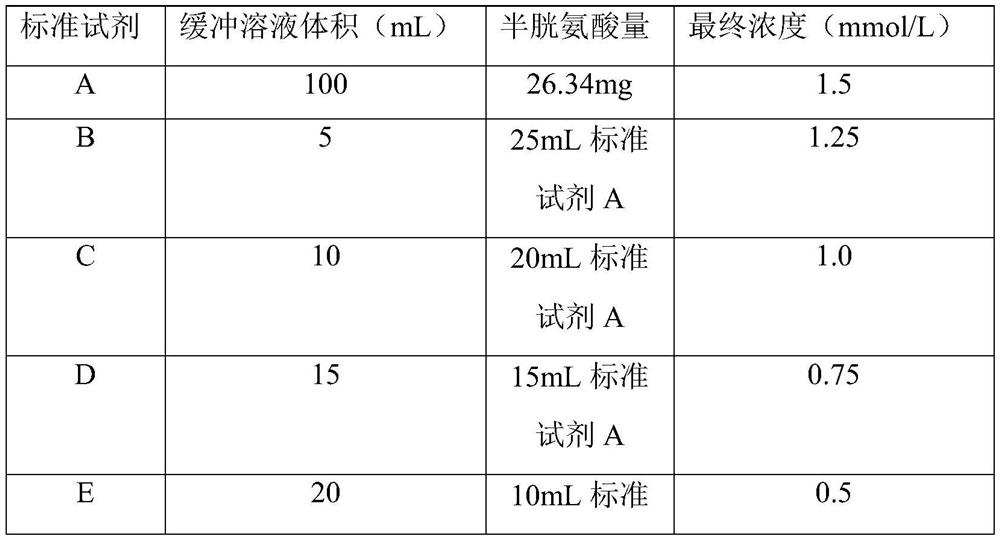
Sulfhydrylated hyaluronic acid as well as preparation method and application thereof
A technology of hyaluronic acid and sulfhydrylation, which can be used in biochemical equipment and methods, cosmetic preparations, cosmetics, etc., and can solve the problems of cross-linking agent residue and influence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Weigh 21g of sodium hyaluronate with a molecular weight of 250kDa, add 800mL of ethanol with a mass fraction of 75%, stir evenly, swell for 1h, filter, add 800mL of ethanol with a mass fraction of 75% to the filter cake, and adjust the pH of the solution with hydrochloric acid to 3.0, stirred, filtered, repeated use of 75% ethanol with a pH value of 3.0 to stir and replace 5 times, filtered, added 300 mL of water to dissolve, adjusted the pH of the solution to 7.0 with tetrabutylammonium hydroxide (TBA-OH), and freeze-dried 26 g of hyaluronic acid tetrabutylammonium salt (HA-TBA) was obtained.
[0043] Weigh 3.0g of HA-TBA, dissolve it in 300mL DMSO, add 6.67g 3,3'-dithiodipropionic acid (DTDP), 1.93g 4-dimethylaminopyridine (DMAP) and 2.0mL dicarbonate tert-butyl ester (Boc 2 0), stirred and reacted for 30h at 45°C, the reaction solution was packed into a dialysis bag (molecular weight cut-off was 3500Da), dialyzed with 0.2wt% sodium chloride solution, and the dialysa...
Embodiment 2
[0046] Weigh 21g of sodium hyaluronate with a molecular weight of 1200kDa, add 1000mL of acetone with a mass fraction of 70%, stir evenly, swell for 1h, filter, add 1000mL of acetone with a mass fraction of 70% to the filter cake, and adjust the pH of the solution with hydrochloric acid to 2.5, stirred, filtered, repeated use of 70% acetone with a pH value of 2.5 to stir and replace 5 times, filtered, added 300 mL of water to dissolve, adjusted the pH of the solution to 7.5 with tetrabutylammonium hydroxide (TBA-OH), and freeze-dried 28.2 g of HA-TBA was obtained.
[0047] Weigh 3.0g of HA-TBA, dissolve it in 600mL of DMSO, add 6.67g of 3,3'-dithiodipropionic acid (DTDP), 1.93g of 4-dimethylaminopyridine (DMAP) and 2.0mL of dicarbonate tert-butyl ester (Boc 2 0), stirred and reacted for 30h at 45°C, the reaction solution was packed into a dialysis bag (molecular weight cut-off was 3500Da), dialyzed with 0.2wt% sodium chloride solution, and the dialysate was replaced once ever...
Embodiment 3
[0050] Weigh 21g of sodium hyaluronate with a molecular weight of 2000kDa, add 1000mL of ethanol with a mass fraction of 90%, stir evenly, swell for 1h, filter, add 300mL of ethanol with a mass fraction of 90% to the filter cake, and adjust the pH of the solution with hydrochloric acid to 3.5, stirred, filtered, repeated use of 90% ethanol with a pH value of 3.5 to stir and replace 5 times, filtered, added 300 mL of water to dissolve, adjusted the pH of the solution to 6.5 with tetrabutylammonium hydroxide (TBA-OH), and freeze-dried 28.7 g of HA-TBA was obtained.
[0051] Weigh 12.0g of HA-TBA, dissolve it in 600mL DMF, add 25g 3,3'-dithiodipropionic acid (DTDP), 7.72g 4-dimethylaminopyridine (DMAP) and 8.0mL di-tert-dicarbonate Butyl ester (Boc 2 0), stirred and reacted for 30h at 45°C, the reaction solution was packed into a dialysis bag (molecular weight cut-off was 3500Da), dialyzed with 0.2wt% sodium chloride solution, and the dialysate was replaced once every 8h, and th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com