Application of Pubescenoside A in preparation of medicine for preventing and treating myocardial ischemia reperfusion injury
A technology for reperfusion injury and myocardial ischemia, applied in the field of biomedicine, can solve problems such as unsatisfactory results, and achieve the effects of reducing myocardial infarct size and CK-MB level, inhibiting damage, and preserving structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1. Take the roots of Ilex pubescens, extract with 12, 10 and 8 times the concentration of 70% ethanol in turn for 2 hours, combine the ethanol extracts, recycle the ethanol after filtration and concentrate under reduced pressure until there is no alcohol smell, to obtain 2.2 kg of total extract ;
[0032] 2. The obtained total extract is dissolved in water, passed through a D-101 type macroporous resin column, and successively eluted with 30% ethanol, 60% ethanol and 95% ethanol; the collection concentration is 30% % ethanol eluent was recovered under reduced pressure to obtain a thick extract 341g, which was passed through a 200-300 mesh silica gel column, using chloroform-methanol as a solvent, with a gradient of 100:1-0:1 in chloroform:methanol Carry out elution; collect the eluent with chloroform:methanol ratio of 4:1, concentrate and pass through ODS column, use methanol:water 3:2 as eluent for elution, collect and concentrate the eluent and pass through Sephadex L...
Embodiment 2
[0034] Identification of the hollylic phenylpropanoid Pubescenoside A (PBA) prepared by the preparation method of Example 1.
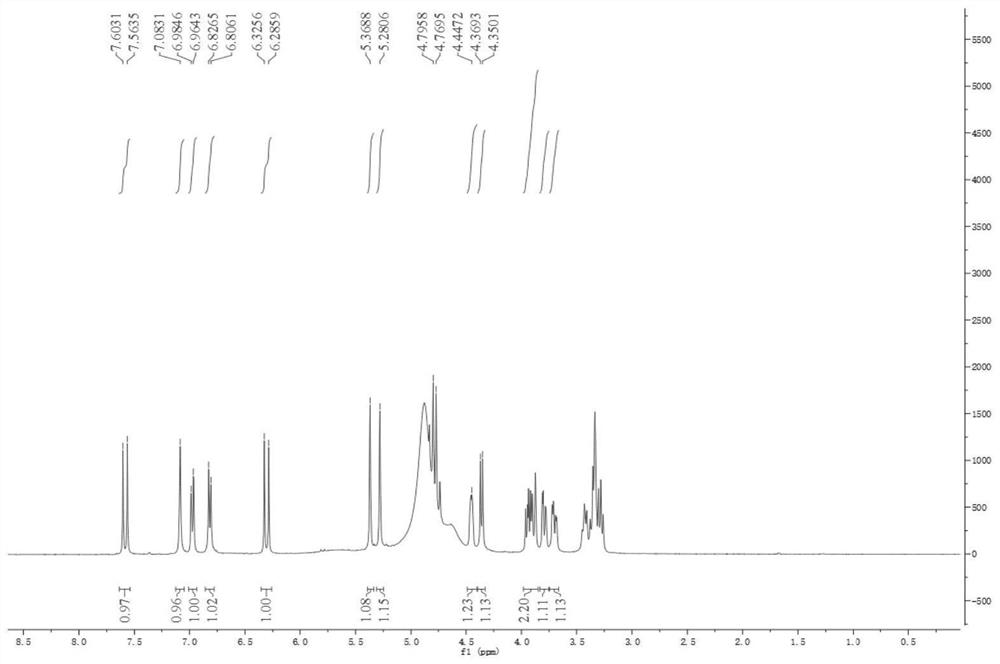
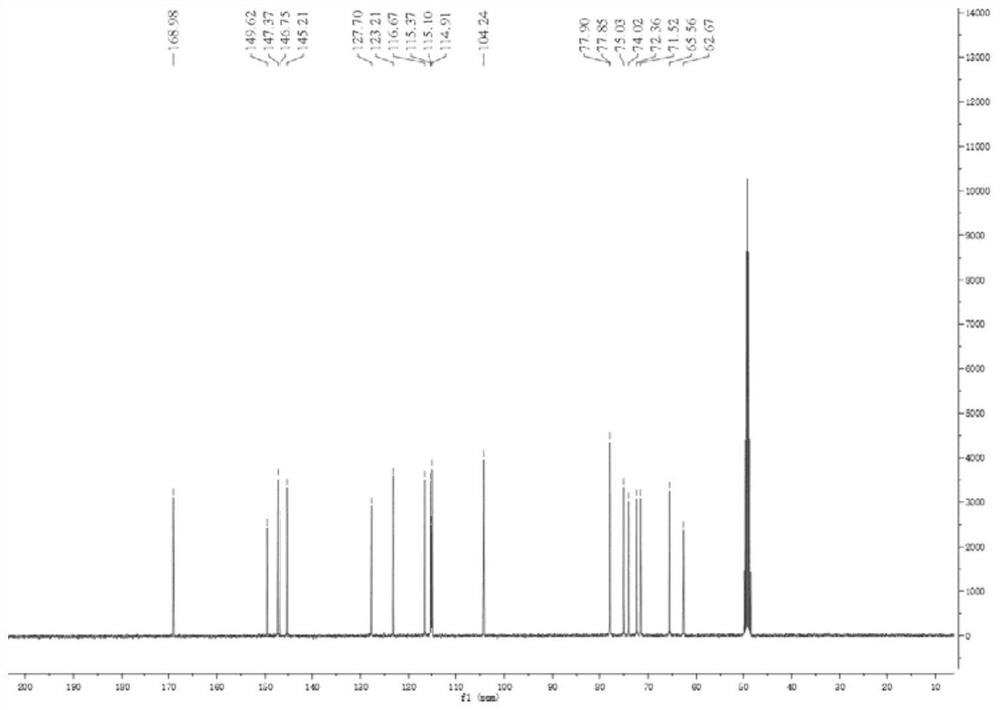
[0035] Light yellow oil, mass spectrum HR-ESI-ME m / z:443.1548[M+H] + . combine 1 H NMR (with figure 1 )with 13 CNMR (attached figure 2 ) deduces that its molecular formula is C 20 h 26 o 11 , degree of unsaturation Ω=8. IR spectrum shows hydroxyl absorption (3338cm -1 ), carbonyl absorption (1688cm -1 ) and benzene ring absorption (1599, 1520, 1446cm -1 ). 1 δ in H NM spectrum H 7.60(1H,d,J=15.8Hz) and δ H 6.33 (1H, d, J = 15.9Hz) is a group of trans-alkene hydrogen proton signals. δ H 7.08(1H,d,J=1.8Hz), δ H 6.96(1H,dd,J=1.8,8.2Hz), δ H6.83 (1H, d, J=8.2Hz) is the ABX system on the benzene ring, which are the hydrogen proton signals of H-2, H-5, and H-6, respectively. δ H 5.37(1H,s) and δ H 5.37(1H,s) is the signal of two protons on another double bond. δ H 4.37 (1H, d, J=7.7Hz) is the terminal hydrogen proton signal of glu...
Embodiment 3
[0040] On the OGD / R model of rat cardiomyocyte H9c2 and the LAD-induced mouse myocardial ischemia-reperfusion model, it is verified that the compound can protect cardiomyocytes and have a protective effect on the heart.
[0041] 1. Determination of cell viability
[0042] Cell viability was determined by MTT assay. RAW264.7 and H9c2 cells (with or without OGD / R treatment) were treated with different doses of PBA for 48 hours. At the end of the experiment, MTT at a concentration of 0.5 mg / mL was added to each well and incubated at 37°C for 3 hours. After 3 hours, the supernatant was discarded, and 150 μL of DMSO was added to each well to dissolve the formazan in the cells, and then the absorbance of the solution at 570 nm was measured by a spectrophotometer (Thermo Fisher Scientific, Massachusetts, USA).
[0043] The results are attached Figure 4 As shown in B, in RAW264.7 and H9c2 cells, PBA did not have any cytotoxicity below 240 μM concentration. Therefore, in the next ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com