Method for constructing in-vitro tissue engineering cartilage
A technology of tissue engineering and constructs, applied in the field of bionic scaffolds, can solve the problems of loss of cartilage phenotype, hypertrophy or calcification of chondrocytes, a large amount of fibrotic cartilage, etc., and achieve the effect of promoting phenotype maintenance and growth of chondrocytes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
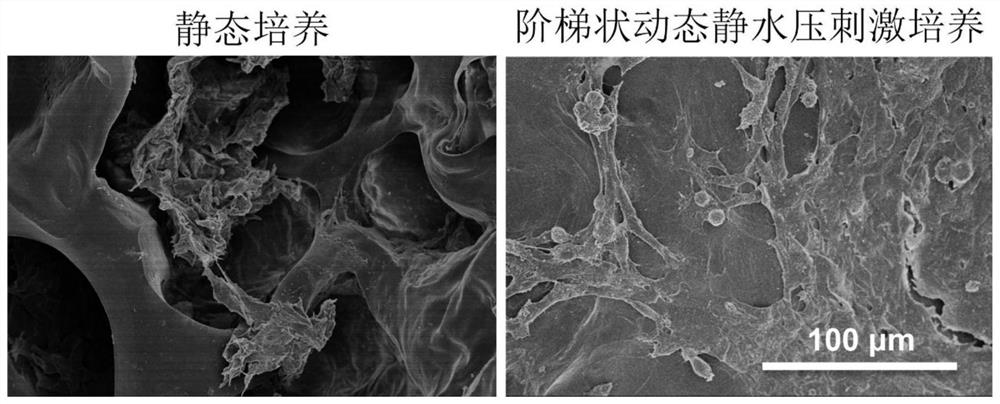
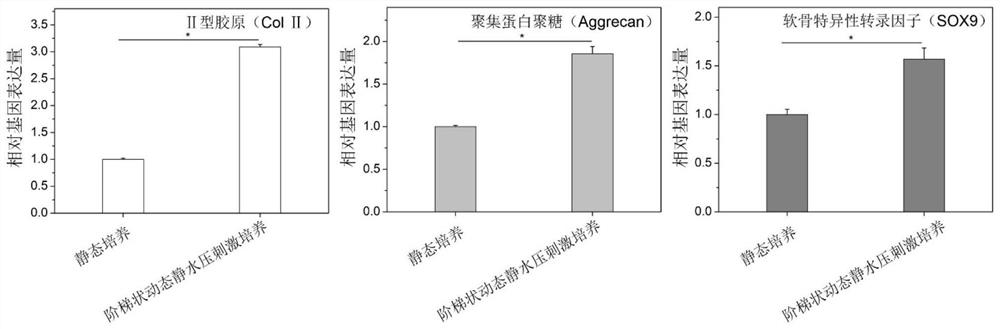
[0034] A method for constructing tissue engineered cartilage in vitro, the specific steps are:
[0035] (1) Preparation of cartilage biomimetic scaffold;
[0036] (1.1) Preparation of raw materials;
[0037] The average diameter is 130nm and the material is the nanofiber bundle of bacterial cellulose; The silk fibroin aqueous solution with the concentration of 6wt%; The H concentration of 490mM 2 o 2 Aqueous solution; HRP solution with a concentration of 1000U / ml (HRP is dissolved in PBS buffer to obtain);
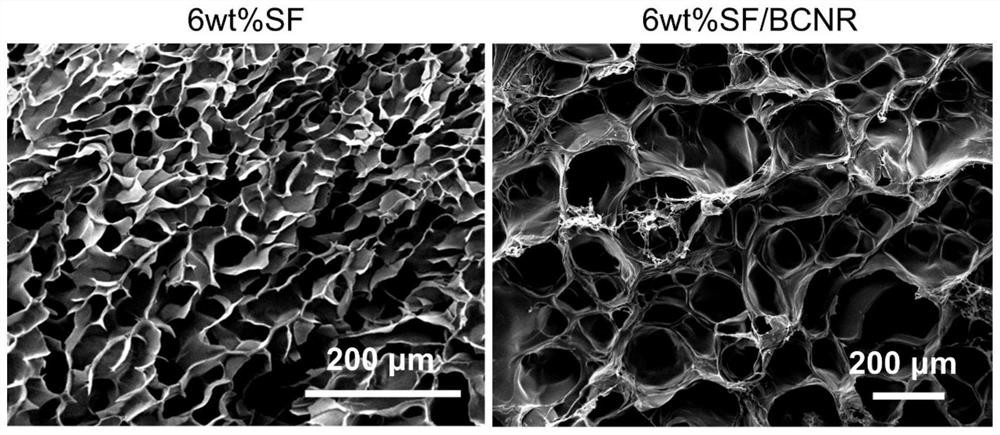
[0038] (1.2) Mix the nanofiber bundles and the silk fibroin aqueous solution to obtain a mixed solution; in the mixed solution, the weight ratio of the silk fibroin and the nanofiber bundles is 95:5;
[0039] (1.3) Add H to the mixture in sequence 2 o 2 Aqueous solution and HRP solution form a crosslinking system; H 2 o 2 The addition of the aqueous solution accounts for 5vol% of the volume content of the crosslinking system; the addition of the HRP solution account...
Embodiment 2
[0054] A method for constructing tissue engineered cartilage in vitro, the specific steps are:
[0055] (1) Preparation of cartilage biomimetic scaffold;
[0056] (1.1) Preparation of raw materials;
[0057] Nanofiber bundles with an average diameter of 30nm and a material made of polyvinyl alcohol; a concentration of 5wt% elastin in water; a concentration of 490mM H 2 o 2 Aqueous solution; HRP solution with a concentration of 1000U / ml (HRP is dissolved in PBS buffer to obtain);
[0058] (1.2) Mix the nanofiber bundles with the elastin aqueous solution to obtain a mixed solution; in the mixed solution, the weight ratio of the elastin and the nanofiber bundles is 95:5;
[0059] (1.3) Add H to the mixture in turn 2 o 2 Aqueous solution and HRP solution form a crosslinking system; H 2 o 2 The addition of the aqueous solution accounts for 3vol% of the volume content of the crosslinking system; the addition of the HRP solution accounts for 4vol% of the volume content of the ...
Embodiment 3
[0069] A method for constructing tissue engineered cartilage in vitro, the specific steps are:
[0070] (1) Preparation of cartilage biomimetic scaffold;
[0071] (1.1) Preparation of raw materials;
[0072] Nanofiber bundles with an average diameter of 100nm and made of polylactic acid; a gelatin aqueous solution with a concentration of 12wt%; a concentration of 490mM H 2 o 2 Aqueous solution; HRP solution with a concentration of 1000U / ml (HRP is dissolved in PBS buffer to obtain);
[0073] (1.2) Mix the nanofiber bundles with the gelatin aqueous solution to obtain a mixed solution; in the mixed solution, the weight ratio of the gelatin and the nanofiber bundles is 95:5;
[0074] (1.3) Add H to the mixture in sequence 2 o 2 Aqueous solution and HRP solution form a crosslinking system; H 2 o 2 The addition of the aqueous solution accounts for 6vol% of the volume content of the crosslinking system; the addition of the HRP solution accounts for 5vol% of the volume content...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
| Compressive strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com