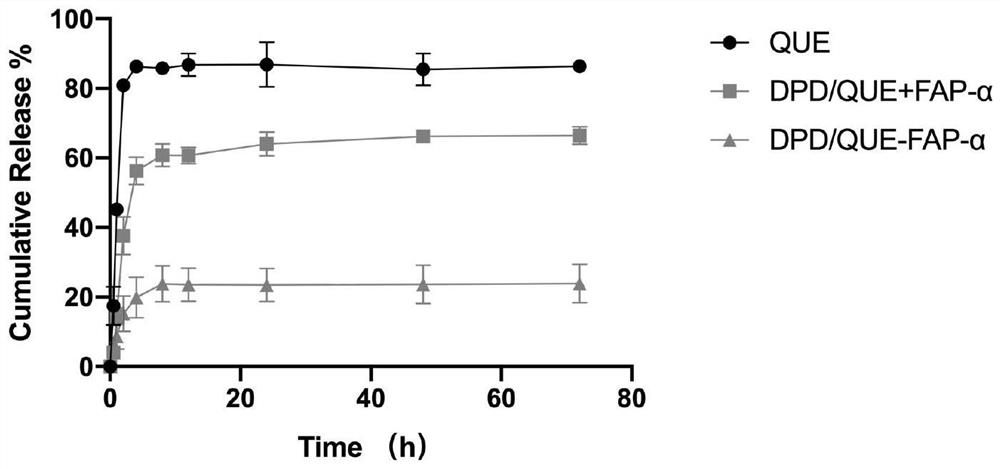
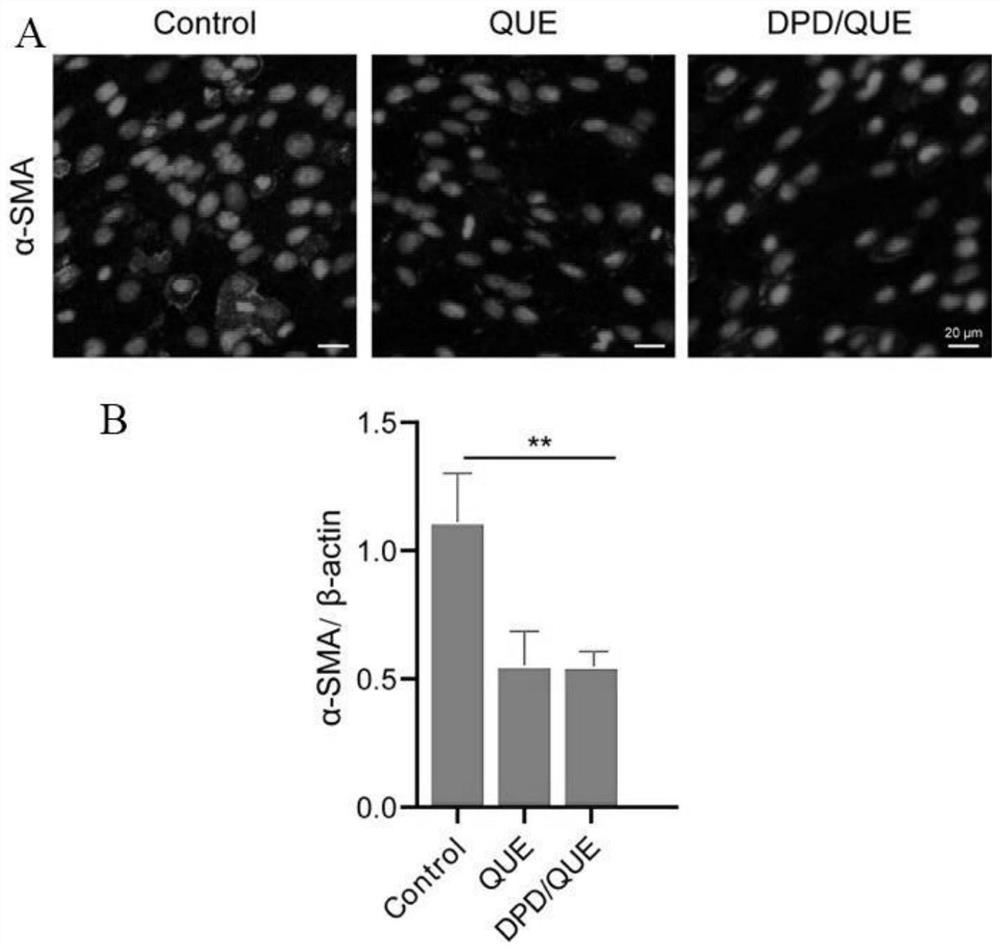
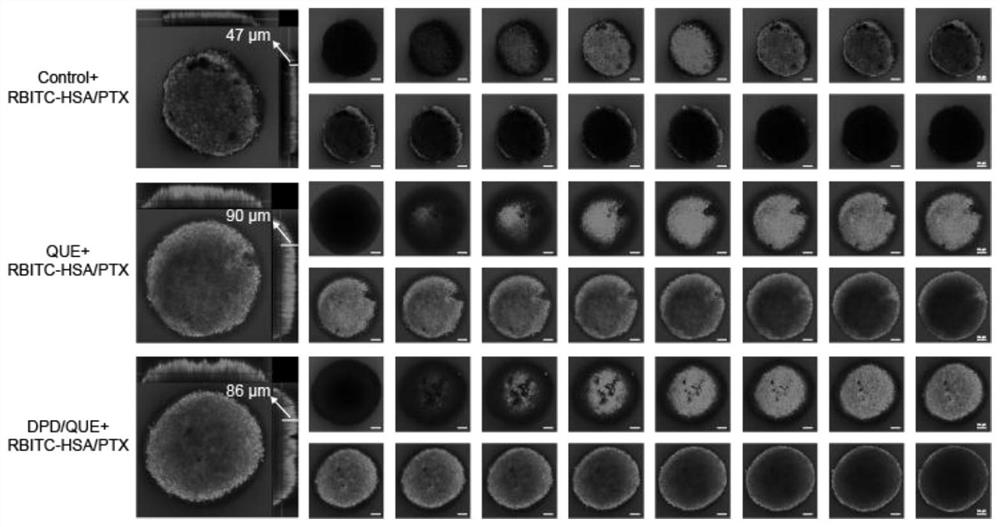
Amphiphilic dextran derivative carrier targeting tumor-associated fibroblasts and preparation and application of pharmaceutical composition of amphiphilic dextran derivative carrier
A technology related to fibroblasts and tumors, which is applied in the field of preparation and application of amphiphilic dextran derivative carriers and their pharmaceutical compositions, and can solve problems such as lack of responsiveness, slow drug release speed, and hindrance to drug efficacy , to achieve the effects of improving solubility, promoting penetration, good biocompatibility and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044]Carboxylated glucan-FAPα response peptide - octade amine (DEX-PEPTIDE-C18The preparation method is as follows:
[0045](1) 2000 mg of glucan was dissolved in dimethyl sulfoxide to obtain a dextran solution; 380 mg of glutanedhydride, 10 mgDMAP was added to the glucan solution, and at normal temperature reacted for 24 h. The reaction liquid was added dropwise to the excess of icy ethanol to form a precipitate, and the precipitate was collected three times using ice ethanol, and after reconstituted, dialysis (MWCO = 7000) 72h, lyophilized, ie, carboxylated glucan intermediate (DEX- COOH);
[0046](2) Take 132 mgboc protected peptide connection arms BOC-SER-GLY-PRO, dissolved in 10 mLDMF; 108 mg of octade amine, 55 mGhobt and 77 mgedc, reaction for 24 h, mixed with reaction mixture, mixed with reaction liquid, extract of ethyl acetate The three times were washed three times with dilute hydrochloric acid, sodium hydrogencarbonate solution and saturated brine. After filtration of anhydro...
Embodiment 2
[0049]The preparation method of carboxylated glucan-FAPα response peptide-peptide-doca is as follows:
[0050](1) 2000 mg of glucan was dissolved in dimethyl sulfoxide to obtain a dextran solution; 334 mg of butyleneic anhydride, 10 mgDMAP was added to the glucan solution, and the temperature reaction was at 24 h. The reaction liquid was added dropwise to the excess of icy ethanol to form a precipitate, and the precipitate was collected three times using ice ethanol, and after reconstituted, dialysis (MWCO = 7000) 72h, lyophilized, ie, carboxylated glucan intermediate (DEX- COOH);
[0051](2) 1000 mg of deoxycholic acid (DOCA) was dissolved in 20 ml of tetrahydrofuran, and 381 mGNHS and 628 mg DCC were added. After 12 h, the white precipitate was removed, and the additional cyclohexane was added to produce a precipitate, and the white precipitate was collected by filtration. Cholic acid-activated ester intermediate; Take 1000 mg of the activated ester intermediate dissolved in 5 mln, N-di...
Embodiment 3
[0055]Carboxylated glucan-FAPα response peptide-lauryl (DEX-PEPTIDE-C12The preparation method is as follows:
[0056](1) 2000 mg of glucan was dissolved in dimethyl sulfoxide to obtain a dextran solution; 427 mg of adipic anhydride, 10 mgDMAP was added to the glucan solution, and the temperature was attenuated for 24 h. The reaction liquid was added dropwise to the excess of icy ethanol to form a precipitate, and the precipitate was collected three times using ice ethanol, and after reconstituted, dialysis (MWCO = 7000) 72h, lyophilized, ie, carboxylated glucan intermediate (DEX- COOH);
[0057](2) Take 167 mgboc protected peptide connection arms BOC-ILE-GLY-PRO-ALA, dissolved in 10 mLDMF; 74 mg of lauryride, 55 mgHobt and 77 mgedc, reaction for 24 h, mixed with reaction mixture, mixed with reaction mixture, extract of ethyl acetate The product was washed three times with dilute hydrochloric acid, sodium hydrogencarbonate solution and saturated brine. After deposurizing sodium sulfate and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com