NK cell culture system and application thereof
A technology of NK cells and culture system, applied in cell culture active agents, cell culture supports/coatings, animal cells, etc., can solve the problems of cumbersome operation and high cost of the culture process, and achieve a safe preparation method, low cost, and low cost. therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1 NK cell culture system
[0037]1. Coating antibody: 96.5% PBS, 0.5% anti-CD3 mAb, 1% anti-CD137 mAb, 0.5% anti-CD161 mAb, 1% anti-CD16 mAb, 0.25% anti-HER2 mAb and 0.25% anti-PD -L1 mAb.
[0038] 2. NK cell activating factor: 98.25% PBS, 1% IL-2, 0.02% IL-7, 0.02% IL-9, 0.03% IL-12, 0.05% IL-15, 0.5% IL-18, 0.03% IL -21, 0.05% IFN-alpha and 0.05% IFN-lamda.
[0039] 3. NK cell basal medium: 99.5% serum-free medium (RPMI1640), 0.1% vitamin mixture (A / B / C / D / E), 0.2% polysaccharide sulfate and 0.2% tea active polysaccharide.
[0040] 4. NK cell activation medium contains: 89.488% NK cell basal medium, 0.01% lipopolysaccharide, 0.002% resveratrol, 0.5% NK cell activating factor and 10% autoinactivated plasma.
[0041] 5. NK cell expansion medium: NK cell basal medium and 500IU / mL IL-2.
Embodiment 2
[0042] In vitro culture expansion of embodiment 2 NK cells
[0043] 1. Antibody coating: After diluting the coated antibody 1:5 with normal saline, add it to a T75 cell culture flask. After standing overnight at 4°C or incubating at room temperature for 2 hours, slowly decant to remove the supernatant, take 10 mL of normal saline to wash the culture bottle once, slowly decant the normal saline before use.
[0044] 2. Autologous Plasma Preparation and Peripheral Blood Mononuclear Cell Isolation
[0045] 1) Centrifuge the anticoagulated whole blood at 2000rpm at room temperature for 10min. Take the supernatant, and place the supernatant in a 56°C water bath for 30 minutes to inactivate. Then, centrifuge at 3500 rpm for 10 min at room temperature. The supernatant was taken as inactivated autologous plasma.
[0046] 2) After diluting the lower blood layer with 0.9% sodium chloride injection 1:1, add it to the top of the lymphatic separation liquid, the ratio of the diluent to ...
experiment example 1
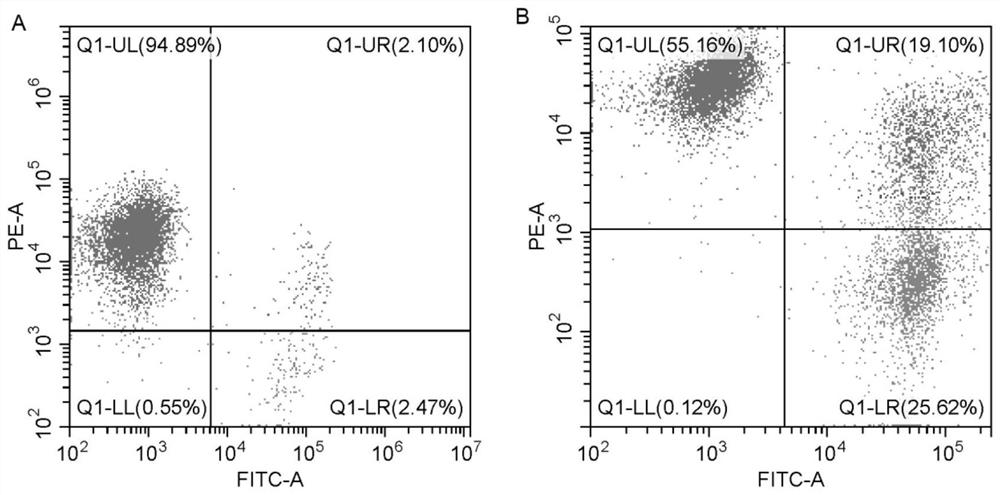
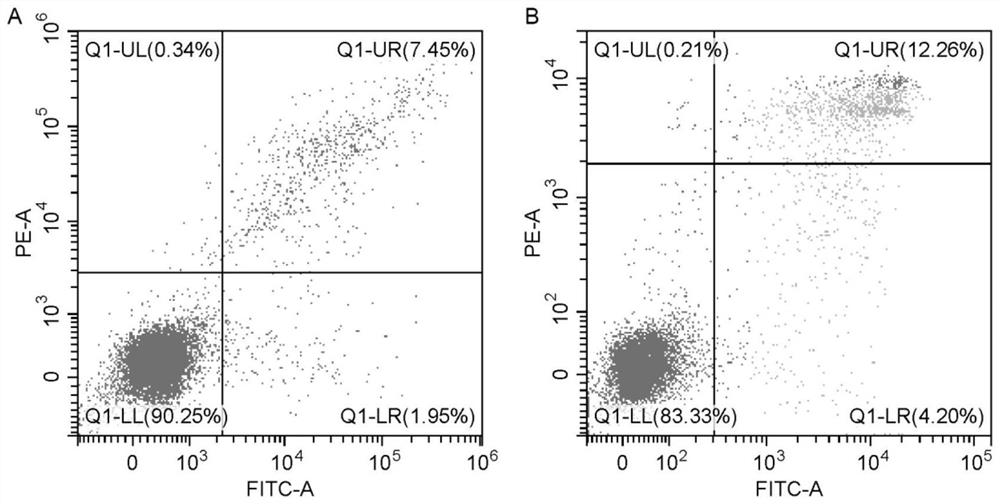
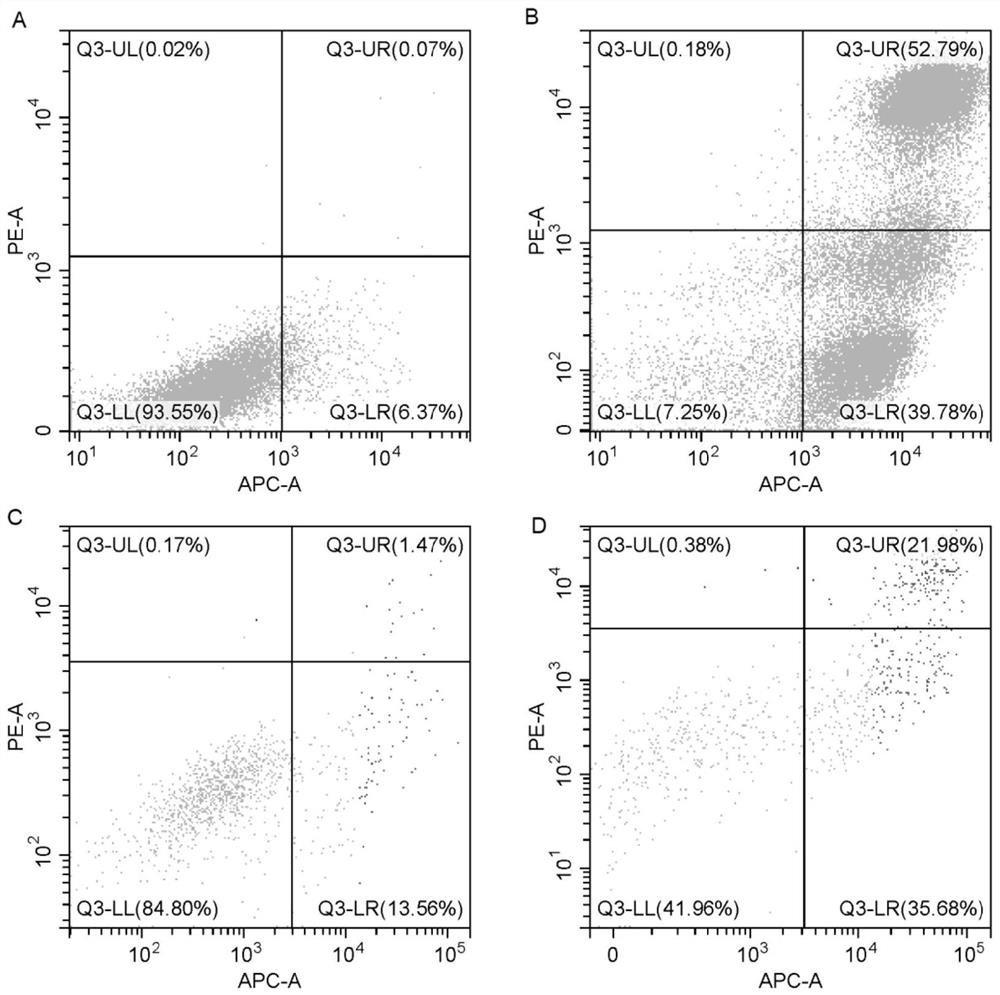
[0057] Experimental example 1 Cell number and activity detection
[0058] The cell suspension prepared in Example 2 and Comparative Example 1 was mixed with 0.2-0.4% trypan blue solution 1:1, and counted by countstar automatic cell counter. The test results are shown in Table 1 below.
[0059] Table 1
[0060] Test items Survival rate cell number Example 2 93% 6.04×10 9
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com