O/W type entecavir microemulsion oral liquid and preparation method of microemulsion oral liquid
A technology of microemulsion oral liquid and entecavir, which is applied in the field of O/W type entecavir microemulsion oral liquid and its preparation field, can solve the problems of low bioavailability of entecavir, poor water solubility, etc. good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
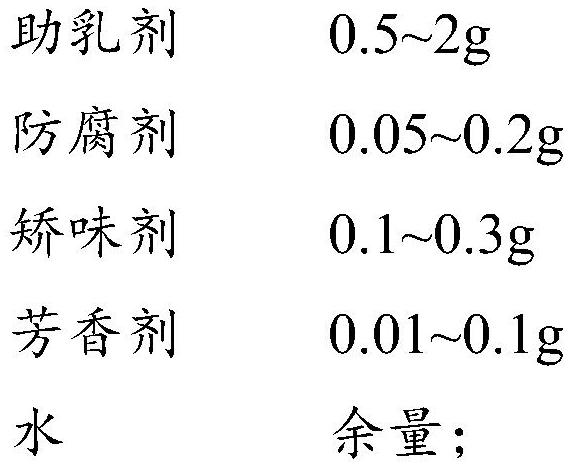
[0027] A kind of O / W type entecavir microemulsion oral liquid, by 100mL, its component composition is as follows:
[0028]
[0029]
[0030] The emulsifier is glyceryl caprate, and the emulsifier is beeswax.
[0031] The preparation method of this O / W type entecavir microemulsion oral liquid is as follows:
[0032] (1) Add entecavir into soybean oil and stir to dissolve;
[0033] (2) Add emulsifier, co-emulsifier and butyl p-hydroxybenzoate to the solution of step (1), stir evenly, then add 20% to 30% of the prescribed amount of water, stir at 10000r / min for 5min to form colostrum;
[0034] (3) Pour the colostrum in step (2) into a homogenizer, add sucralose, lemon essence and the remaining water, and disperse homogeneously at 20000r / min for 10min to form a stable O / W emulsion. Seal and sterilize to obtain O / W type entecavir microemulsion oral liquid.
Embodiment 2
[0036] A kind of O / W type entecavir microemulsion oral liquid, by 100mL, its component composition is as follows:
[0037]
[0038] The emulsifier is glyceryl caprate, and the emulsifier is beeswax.
[0039] The preparation method of this O / W type entecavir microemulsion oral liquid is as follows:
[0040] (1) Add entecavir to corn oil and stir to dissolve;
[0041] (2) Add emulsifier, co-emulsifier and butyl p-hydroxybenzoate to the solution of step (1), stir evenly, then add 20% to 30% of the prescribed amount of water, stir at 20000r / min for 3min to form colostrum;
[0042] (3) Pour the colostrum in step (2) into a homogenizer, add acesulfame-K, stevia, sweet orange essence and the rest of water, and disperse homogeneously at 10000r / min for 15min to form a stable O / W type emulsion, potting, and sterilizing to obtain O / W type entecavir microemulsion oral liquid.
Embodiment 3
[0044] A kind of O / W type entecavir microemulsion oral liquid, its difference with embodiment 1 is only that the addition of emulsifier and co-emulsion is different, in the present embodiment, the addition of emulsifier caprylic capric acid glyceride is 1.5g, The addition amount of auxiliary emulsifier beeswax is 1.5g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com