Preparation method of chiral alpha-methyl arylethylamine
A methylarylethylamine and chiral technology, which is applied in the field of preparation of chiral α-methylarylethylamine, can solve the problems of being unsuitable for large-scale industrial production, low atom utilization rate, and high production cost, so as to avoid a large amount of The generation of solid waste and isomers, the effect of overcoming the separation problem of column chromatography and reducing the hidden dangers of production safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
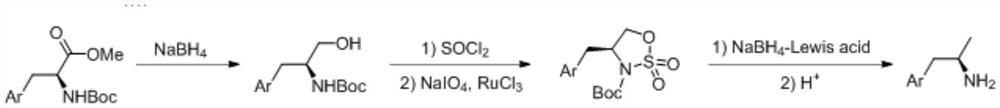
[0054] (1) Add Boc-L-tryptophan methyl ester (318.4g, 1.0mol) into a 5L three-necked flask, add ethanol (2.5L), and add sodium borohydride (95.1g, 2.5mol) at a temperature of 0-10°C , Reaction at 30°C for 10h after addition. Saturated ammonium chloride solution (1.5 L) was added dropwise at 0-5°C under temperature control, and ethanol was distilled off under reduced pressure. Ethyl acetate (2 L) extracted 2 times. The combined organic phases were dried with sodium sulfate, concentrated under reduced pressure, and slurried with n-heptane to obtain 275.7 g of the product Boc-L-tryptophanol with a yield of 95% and a purity of 99.1%.
[0055] (2) Boc-L-tryptophanol (145.0g, 0.5mol) obtained in step (1) was added into a 5L three-necked flask, and dichloromethane (1.5L), triethylamine (111.0g, 1.1mol), Imidazole (102.0 g, 1.5 mol) was added dropwise with thionyl chloride (65.5 g, 0.55 mol) under temperature control at 0-5°C, and the reaction was kept for 4 hours after the addition...
Embodiment 2
[0060] Different from Example 1, in step (3), Lithium Chloride Anhydrous is changed into Zinc Chloride Anhydrous, and all the other are the same.
[0061] The reaction finally yielded 27.1 g of the product with a yield of 78% and a purity of 99.2%.
Embodiment 3
[0063] Different from Example 1, step (3) was changed to add the sulfonamide compound (70.5g, 0.2mol) obtained in step (2) in a 2L there-necked flask, and diethylene glycol dimethyl ether (1L) was added , Sodium borohydride (15.2g, 0.4mol), anhydrous lithium chloride (54.4g, 0.4mol), react at 80°C for 24h. The reaction solution was added to 4M hydrogen chloride-ethyl acetate solution (150 mL) under temperature control at 5-10°C, and reacted at 30°C for 12h. After precipitation under reduced pressure, saturated sodium bicarbonate solution was added, extracted twice with ethyl acetate (400 mL), dried over sodium sulfate, and concentrated under reduced pressure to obtain a crude product, which was recrystallized from ethyl acetate and n-heptane. Everything else is the same.
[0064] The reaction finally yielded 23.8 g of the product, with a yield of 68% and a purity of 98.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com