Pyrene unit-containing organic fluorescent material with aggregation-induced emission as well as preparation method and application of pyrene unit-containing organic fluorescent material
A technology of aggregation-induced luminescence and fluorescent materials, applied in the field of fluorescent inks, can solve the problems of easy oxidative decomposition loss, poor thermal stability, fluorescence quenching, etc., and achieve the effects of good biocompatibility, good thermal stability, and high color purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] The preparation of embodiment 1 carbazole biphenylacetonitrile
[0052] Under the condition of nitrogen protection, add p-benzocarbazole borate and p-bromophenylacetonitrile (molar ratio is 1: (1~3)), potassium carbonate, tetrakis (triphenylphosphine) palladium into the double-necked flask, Then add 10-20mL of toluene, ethanol and water, stir vigorously, keep the temperature at 90°C, reflux for 24h, cool to room temperature, extract, wash and filter to obtain the primary product, separate by chromatography column or recrystallize to obtain carbazole biphenyl Acetonitrile, its molecular structure is shown in 1a in formula (1), its synthetic route is shown in formula (1), and its productive rate is about 61%.
[0053]
Embodiment 2
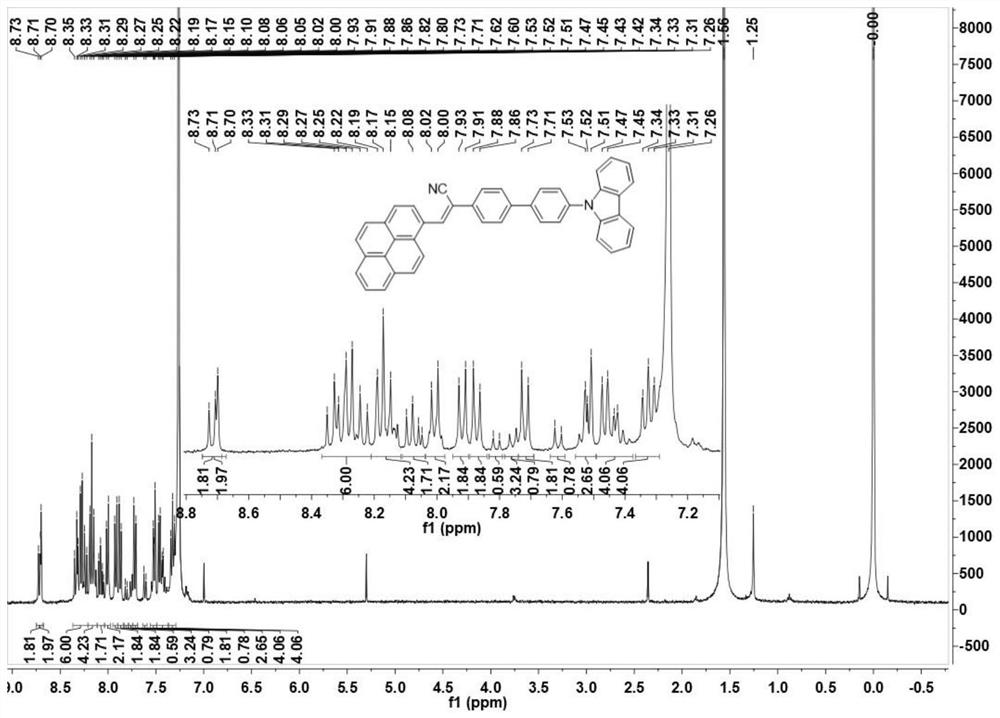
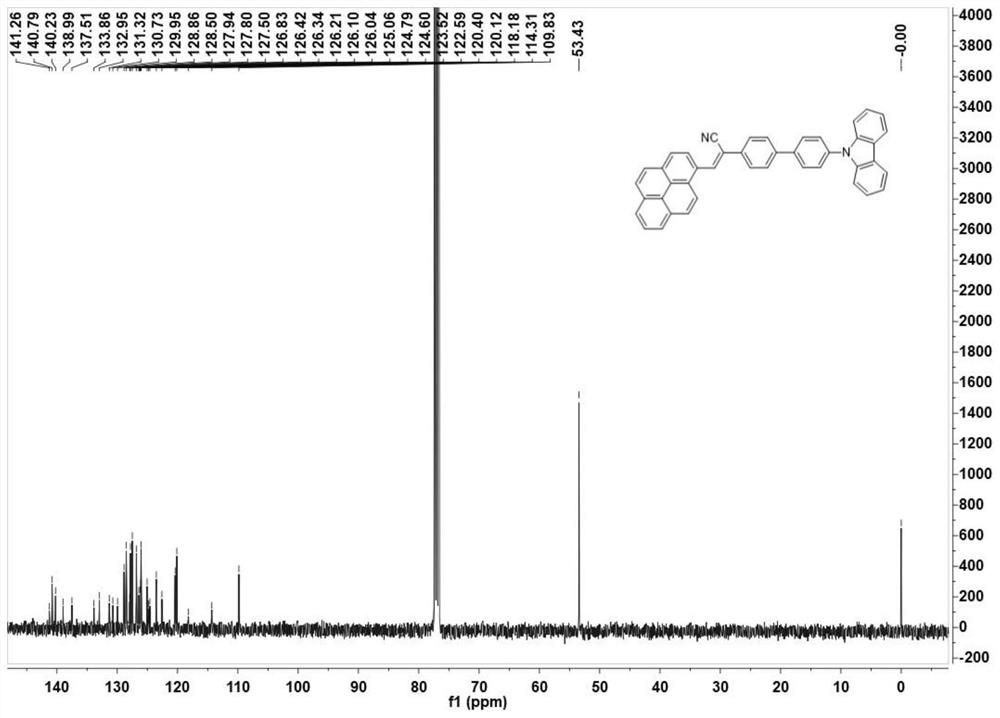
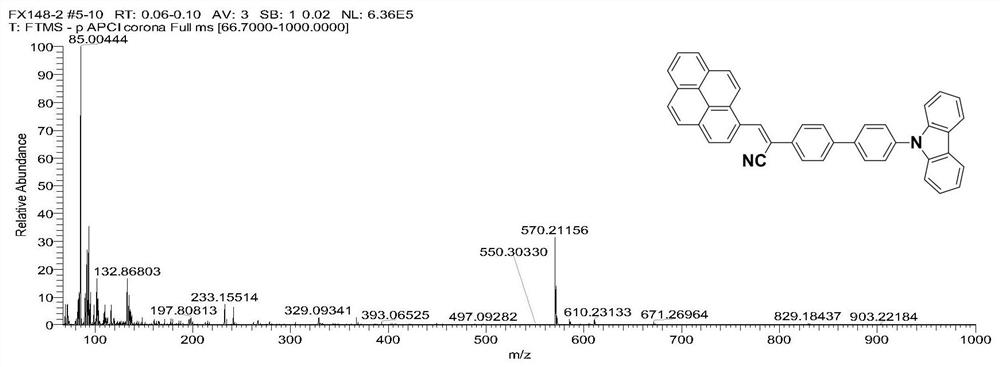
[0054] Example 2 Preparation of 2-(4'-(9H-carbazole)-[4-(1,1'-biphenyl)]-3-(1-pyrenyl)acrylonitrile (Py-2Ph-Cz)
[0055] Under the condition of nitrogen protection, the carbazole biphenylacetonitrile and 1-pyrene formaldehyde and potassium tert-butoxide prepared in Example 1 are added in the double-necked bottle (the molar ratio is 1: (1~3): 3), and then Add 10-20mL of absolute ethanol, stir vigorously, keep the temperature at 90°C, reflux for 24h, cool to room temperature, wash with ethanol and suction filter to obtain the initial product, and further recrystallize in a mixed solvent of dichloromethane and n-hexane to obtain 2- (4'-(9H-carbazole)-[4-(1,1'-biphenyl)]-3-(1-pyrenyl)acrylonitrile (Py-2Ph-Cz), its molecular structure is as follows: As shown in 2a in 2), its synthetic route is shown in formula (2), and its yield is about 74%.
[0056]
[0057] figure 1 Obtain the Py-2Ph-Cz for embodiment 2 1 H NMR chart. figure 2 The 13CNMR pattern of Py-2Ph-Cz was obtained...
Embodiment 3
[0058] Example 3 Preparation of 1-(4-benzaldehyde)pyrene
[0059] Under the condition of nitrogen protection, add 1-bromopyrene and 4-formylphenylboronic acid (the molar ratio is 1:(1~3)), potassium carbonate, tetrakis-(triphenylphosphine)-palladium into the double-necked flask , then add 10-20mL of toluene, ethanol and water, stir vigorously, keep the temperature at 90°C, reflux for 24h, cool to room temperature, extract, wash and filter to obtain a crude product, separate by chromatography column or recrystallize to obtain 1-(4 -benzaldehyde group)pyrene, its molecular structure is shown in 3a in formula (3), its synthetic route is shown in formula (3), and its yield is about 51%.
[0060]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com