Fully continuous flow preparation method of 3-chloro-4-amyl oxoacetate
An amyl acetate, fully continuous technology is applied in the preparation of carboxylate, the preparation of organic compounds, the preparation of ester groups and hydroxyl groups, etc. The effect of shortened time, high degree of automation and improved reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
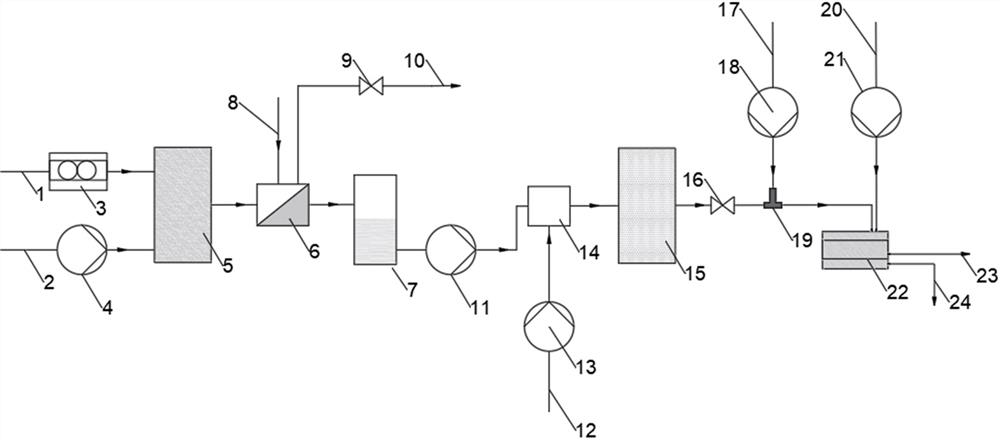
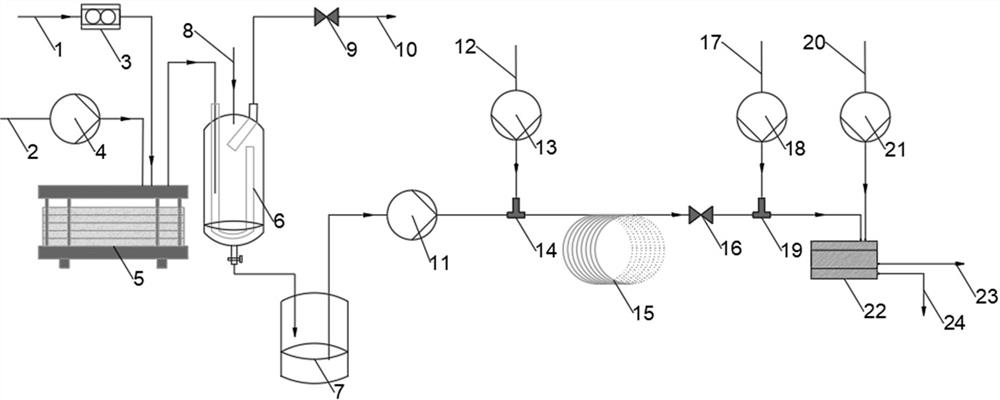
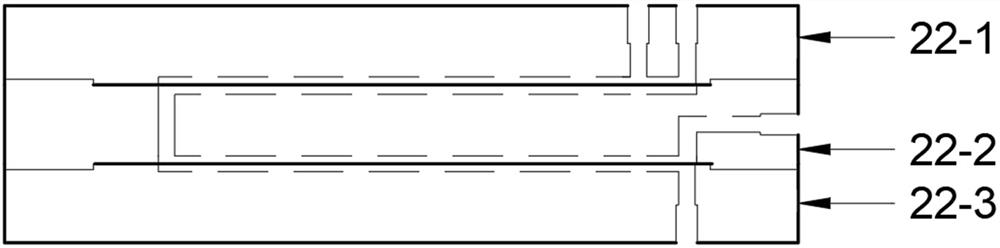
[0059]The acetylbutyrolactone liquid and the chlorine gas were simultaneously delivered to the Protrix microchannel reactor (the reaction volume was 4.2 ml, and the diameter of the microchannel was 2 mm), and the flow ratio of the acetylbutyrolactone liquid and the chlorine gas was adjusted so that the substrate acetylbutyrolactone The molar ratio of lactone (3) to chlorine is 1:1.3, the flow rate of acetylbutyrolactone liquid is 1.0 ml / min, the back pressure value of the back pressure valve is set to 0.2 Mpa, and the temperature in the Protrix microchannel reactor is controlled 25 ℃, after 30 seconds of reaction (that is, the residence time of the mixed reaction material in the microchannel reactor is 30 seconds), the mixed reaction material flows out from the outlet of the microchannel reactor, and after the gas component is separated by the gas-liquid separator, Collected in the product buffer tank. The conversion rate of sampling detection acetylbutyrolactone is 100%. The...
Embodiment 2
[0061] This embodiment is the same as Example 1, except that the only difference is that the micro-mixer before the acylation reaction in this embodiment is a T-type micro-mixer. In this example, the substrate acetylbutyrolactone was also completely converted, and the yield of the obtained product 3-chloro-4-oxoacetate amyl ester (1) was 89%, and the purity was 93% (GC).
Embodiment 3
[0063] This embodiment is the same as Example 1, except that the microchannel reactor used for the chlorination reaction in this embodiment is a tubular microchannel reactor with a volume of 5 milliliters and an internal diameter of 0.8 millimeters. In this example, the substrate acetylbutyrolactone was completely converted, and the yield of the obtained product 3-chloro-4-oxoacetate amyl ester (1) was 90.1%, and the purity was 96% (GC).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com