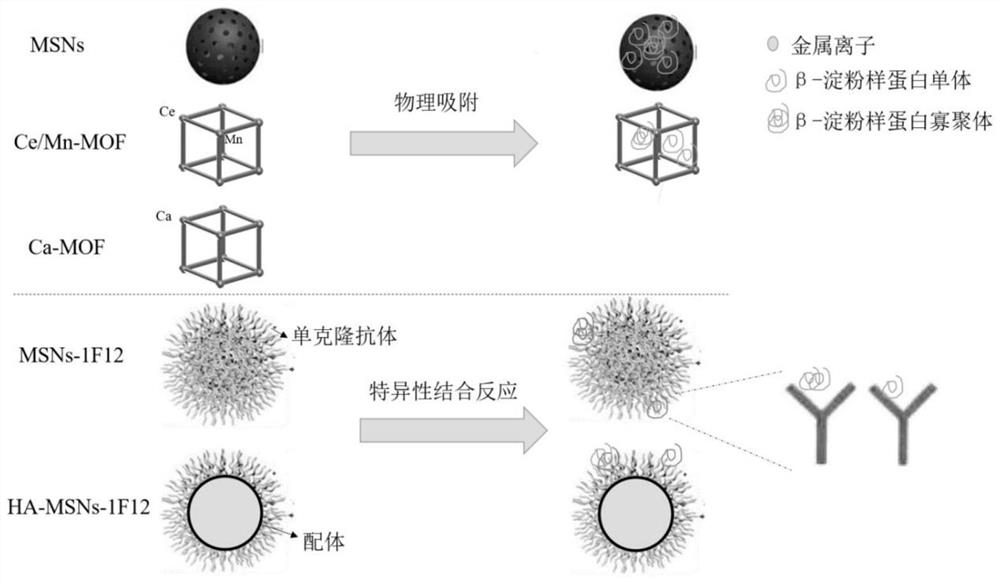
A specific capture carrier for removing β-amyloid protein and its application
An amyloid-specific technology, applied in the direction of immunoglobulin, carrier-bound/immobilized peptide, anti-animal/human immunoglobulin, etc., can solve the problems of neuronal functional defects and the inability to realize Aβ peripheral clearance, etc., to achieve High drug stability, fast special equipment, effect of reducing burden
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
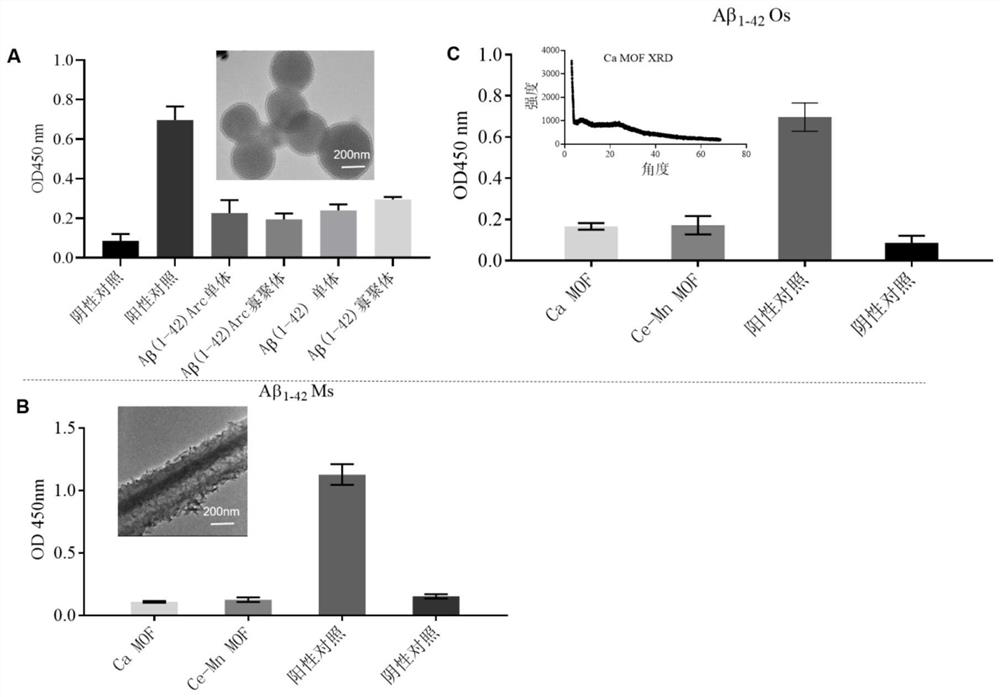
[0038] Example 1: Adsorption of β-amyloid by MSNs and Ca-MOF / Ce-Mn MOF
[0039] 1.1. Synthesis of MSNs and Ca-MOF / Ce-Mn MOF
[0040] Synthesis of MSNs:
[0041] Mix 5mL of Cetyltrimethylammonium chloride (CTAC0.274mmol) and 5mL of triethanolamine (Triethanolamine, TEA 51.1mmol) with magnetic stirring and raise the temperature to 95°C. After 1h, 0.5mL of Ethyl orthosilicate (Tetraethylorthosilicate, TEOS 2.23mmol) was added dropwise and the temperature was maintained to continue magnetic stirring for 1h. Then, MSNs were obtained by centrifugation and washing with ethanol 3 times. The obtained MSNs were stirred in 1% hydrochloric acid ethanol solution at 60° C. for 3 h, repeated three times, and centrifuged to obtain the template removing agent CTAC-MSNs. Its transmission electron microscope image is shown in figure 2 Shown in Figure A.
[0042] Synthesis of Ce-Mn MOF:
[0043] Take 103.89g MnCl respectively 2 4H 2 O and 83.92 g CeCl 3 7H 2 O was dissolved in absolu...
example 2
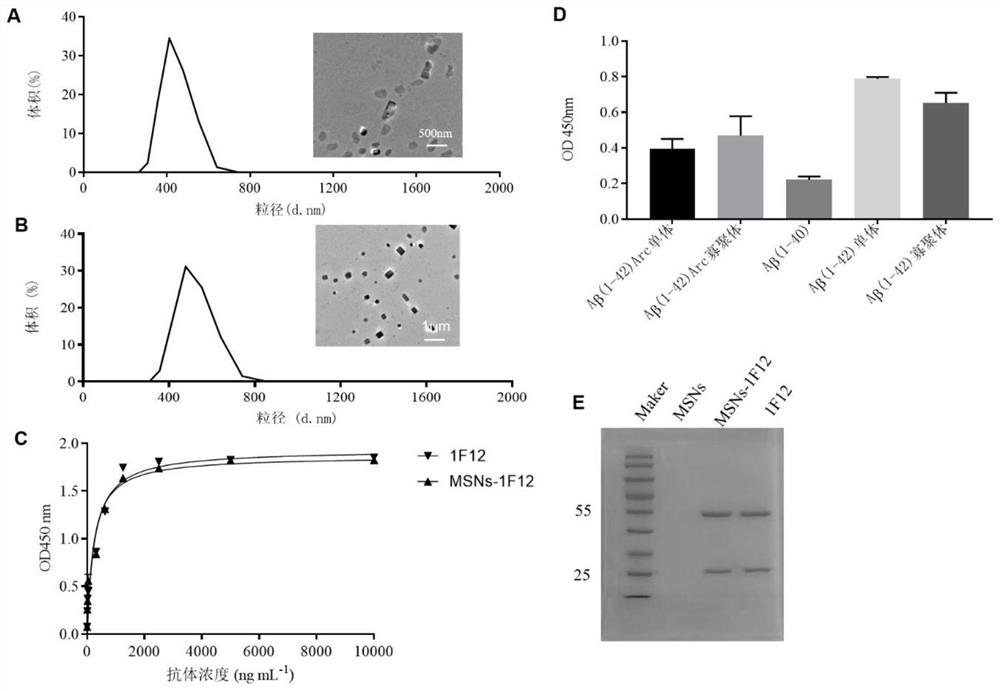
[0049] Example 2: MSN S Clearance of β-amyloid protein in peripheral blood of APP / PS1 mice by -1F12 nanoparticle probe
[0050] 2.1. MSN S Preparation and detection of -1F12 nanoparticle probe
[0051] NHS modified MSNs: 5.4x 10 -6 mol NHS (NHS, N-hydroxysuccinimide, click chemical reaction with amino groups, used for bioconjugation and labeling) was added to 3 mL of CTAB-MSNs samples and reacted for 3 h; centrifuged, washed 3 times with PBS, dispersed in Store in 200 μL DI in the dark.
[0052] Coupling of 1F12 antibody: Mix MSNs-NHS and 1F12 antibody at a ratio of 10:3, and use 0.1M Na 2 CO 3 Adjust the pH value between 8.5 and 9.0, mix the two fully in the state of vortex, and incubate at 4°C for 3 to 5 hours; get MSN S -1F12 Nanoparticle Probe, MSN S -1F12 was dissolved in PBS at a concentration of 1 μg / μL for use; 1F12 was dissolved in PBS at a concentration of 1 μg / μL for use; MSNs-NHS was dissolved in PBS at a concentration of 1 μg / μL for use. MSNs and MSN S - ...
example 3
[0061] Example 3: HA-MSNs-1F12 Nanoparticle Probe Clearance of β-Amyloid in the Peripheral Blood and Brain of APP / PS1 Mice
[0062] 3.1. Preparation of HA-MSNs-1F12 functionalized nanoparticles
[0063] Fe 3 o 4 Preparation of @OA nanoparticles: monodisperse Fe 3 o 4 @OA nanoparticles were prepared by chemical co-precipitation method, under nitrogen flow, 6.56g FeCl 2 4H 2 O (33.5mmol) and 11.30g FeCl 3 6H 2 O (41.8 mmol) was dissolved in 80 mL of deionized (DI) water. The solution was heated to 80 °C and stirred vigorously for 0.5 h. 45 mL of ammonium hydroxide was added slowly and the resulting suspension was vigorously stirred for 5 min. 2 mL of OA was added and the mixture was kept at 80 °C for 25 min. The mixture was naturally cooled to room temperature. The black product was collected using a magnet and washed thoroughly with methanol and DI water to remove excess OA. The obtained oleic acid-stabilized monodisperse magnetic nanoparticles (Fe 3 o 4 @OA) dri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com